My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.107 n.10 Madrid Oct. 2015
Fully covered metal stents for the treatment of leaks after gastric and esophageal surgery
Alberto Fernández, Víctor González-Carrera, Carlos González-Portela, Amalia Carmona, Manuel de-la-Iglesia and Santiago Vázquez
Department of Digestive Diseases. Hospital POVISA. Vigo, Pontevedra. Spain
ABSTRACT
Objective: The use of fully covered metal stents (FCMS) for the treatment of benign conditions is increasing. The aim of our study was to assess the efficacy of FCMS in the management of post-operative leaks after gastric or esophageal surgery.
Material and methods: During a three year period (2011-2013), patients who underwent a surgery related with esophageal or gastric cancer and developed a postoperative anastomotic leak treated with FCMS were prospectively included.
Results: Fourteen patients were included (11 men, 3 women), with median age of 65 years. Placement of at least one stent was achieved in 13 patients (93% of cases), with initial closure of the leak in 12 of these 13 cases (92.3%). A final success (after removal of the stent) could be demonstrated in 9 cases (69.2%, intention to treat analysis); stent failed only in one case (7.7%) and there were 3 patients (23.1%) not evaluated because death before stent retrieval (not related with the endoscopic procedure). One stent were used in 9 cases (69.2%), and two in 4 (30.8%). Migration was observed in two cases (15.3%). There were no major complications related with the use of stents. There were no complications related with retrieval.
Conclusions: The placement of FCMS to achieve the leak closure after esophageal or gastric surgery is an effective and probably safe alternative feasible with minor risks.
Key words: Endoscopy. Anastomotic leak. Fully covered self-expandable metal stent. Stents. Treatment.
Introduction
Surgery is the best curative treatment for patients with localized esophageal or gastric cancer, having a clear impact on survival. Nevertheless, gastric and especially esophageal resections are technically challenging procedures associated with morbidity and even mortality. Perioperative surgical complications are mainly due to anastomotic leaks described in 4-27% of patients after radical gastrectomy (1-3) and 5-18% after esophagectomy (4-6). These complications are life-threatening medical emergencies and have a mortality rate up to 30% (2). Minor leaks without sepsis can be managed conservatively but major disruptions require a risky reoperation with important hospital resources consumption and long hospitalization. In order to avoid surgical repairs, many endoscopic techniques such as clipping (7) or fibrin glue application (8) have been proposed as an alternative, with no proven efficacy.
Another endoscopic approach to this problem is the use of self-expandable stents. Several types of stents have been used, generally with good results. Self-expandable plastic stents (SEPS) were used first, presenting migration as their mayor complication (9-18); partially covered self-expanding metal stent (PCMS) were also used with a mayor risk of developing hyperplastic inflammatory tissue through the open mesh of the stent making the removal of the stent difficult (19-25). Subsequently, in an attempt to minimize these complications, fully covered self-expanding metal stents (FCMS) were developed. First reports about its use in benign conditions (including leaks, strictures or perforation) showed good results and a reasonable safety profile (26-38).
The aim of our study was to assess the effectiveness of FCMS (Wallflex, Boston Scientific Corp. Nattick. Massachuset. United States) in the management of post-operative leaks after gastric or esophageal surgery.
Material and methods
Patients
In a prospective study from January 2011 to December 2013, patients who underwent a surgery related with esophageal or gastric cancer in our institution and developed a postoperative anastomotic leak were prospectively eligible to be included in our study. All patients gave informed consent.
Diagnosis and treatment of anastomotic leak
Diagnosis of post-operative leak was made using water soluble contrast at day 5-7, according to our protocol (39), or earlier in cases with clinical symptoms or radiological signs. All patients with signs of leak were endoscopically treated using FCMS. Patients remained in bed in decubitus position for 48 hours. After this period the effectiveness of leak occlusion and the absence of stent migration were evaluated by water soluble contrast swallow. In cases of therapeutic success, feeding was immediately started.
Stent retrieval was performed endoscopically 6-8 weeks after deployment, using also fluoroscopic control, with patients under general anesthesia to avoid risk of aspiration. Rat-tooth forceps was the most common used device, grasping at the proximal end of the stent and pulling out. Finally, complete closure of the leak was verified with radiological contrast study (Fig. 1).
All endoscopic procedures were performed by two endoscopist, with experience in the use of enteral stents.
Demographic data (sex, age, indication of surgery, type of surgery -including laparoscopic or conventional approach-) were collected. Technical success was defined as the placement of the stent in the right location. Initial success was defined as absence of contrast extravasation after stent placement. Final success was defined as complete closure of leak after stent retrieval. Number of stents used, migration, need of relocation, and problems related to extraction were also analyzed. Finally, mortality and its relationship with initial diagnosis or stent complication were included.
Results
The study includes 14 patients (11 men, 3 women) undergoing stent placement, with a median age of 65 years (range 46-77). Table I shows indication ant type of surgery; ten patients (71.5%) had been previously diagnosed of gastric cancer, and 4 patients (28.5%) with esophageal cancer. In one case surgery was performed with palliative intention (esophago-jejunal by pass).
Technical success was achieved in 13 patients (93%). The only case of failure was a patient with a complete anastomotic dehiscence where the identification of the efferent loop was impossible. Initial success was obtained in 12 of 13 patients (92.3%) in which stents were place. Final success was obtained in 9 of these cases (69.2%, intention to treat analysis; stent failed in one case (7.7%) and three patients due to death before stent retrieval (23.1%). These three deaths were not related to stent placement but to progression of the initial neoplasia. Ignoring these three deceased patients, final success was 90%.
In most of the cases only one stent was needed (69.2%) and in four cases two stents were necessary (30.8%) to obtain initial success. Migration was observed in two cases (15.3%) and relocation of the stent was possible in both patients. table II shows case evolution. No major complications related to the use of stents, such as fistula, hemorrhage or perforation occurred. Only one patient referred pain after stent deployment.
Median of days between surgery and insertion of stent was 7 (limits 2-49). Median of days between initial insertion of the stent and retrieval was 44 (limits 12-101). No complications related to retrieval arouse.
Specific characteristics of individual cases are separately commented below:
- Patient number 2 was the only technical failure due to a complete anastomotic dehiscence, needing urgent reoperation.
- Patient number 3 had the upper limit of stent deployment (day 49) due to late presentation of clinical signs of fistula; this patient was also the case with an earlier stent withdrawal due to chest pain (day 12 after insertion). Notwithstanding, a complete closure of the leak was achieved.
- In patient number 7, retrieval of the stent was performed substantially later compared to the rest of cases (101 days). This discrepant result was due to a severe clinical decompensation of a cardiac disorder delaying the procedure.
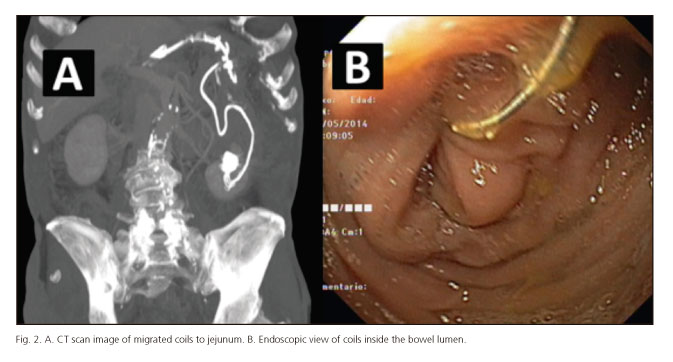
- In patient number 14, initial and final success was achieved. During the period with the stent placed, the patient presented a hypovolemic shock due to an acute hemorrhage originated in an aneurism of the hepatic artery, treated with insertion of coils by a radiologist. Three months after stent retrieval, migration of coils to jejunum adjacent to the anastomosis was observed, causing a new fistula (Fig. 2). Coils were endoscopically retrieval, and a new FCMS was placed also with initial good result. Nevertheless, when stent was retrieval 9 weeks later, persistence of the fistula was observed; then a surgical approach was performed, finding neoplastic local recurrence.
Discussion
In this study we describe our three-year's experience with FCMS for treatment of post-operative leaks in patients with esophageal or gastric cancer. The use of stents for the treatment of benign esophageal conditions such as strictures, post-surgical leaks, iatrogenic or spontaneous perforations is widespread. Nevertheless, the availability of different types of stents may induce doubt which is the best option in each situation (uncovered, partially covered or fully covered metallic or plastic). At the time of the beginning of our study there were no published reports comparing them. Our decision of using FCMS was based on the following criteria. Uncovered metallic stents become embedded into the gut wall and retrieval might be extremely or even impossible. SEPS avoid this risk, but present high rates of migration up to 40% (9-18) in relation to the absence of a uncovered portion and with low radial force. PCMS combine good radial force with an inner silicone membrane that prevents the growth of tissue through the mesh except at their ends; nevertheless, the uncovered portion may also strongly anchor to the wall and safe removal may be complicated (40) with high rates of perforation. To facilitate removal of PCMS, the stent-in-stent technique (placing a SEPS inside the partially covered stent, and removing both stents in two weeks) can be used with good efficacy (41) but increasing the costs significantly. Theoretically, FCMS should not get embedded, should have enough force to reduce migration rate and, from our point of view, the introduction system is simpler than plastic stent delivery, likewise, the retrieval of stent is easier too.
Initial publications about FCMS show good results, with leaks closure ranging from 38% to 100% (26-38). However the interpretation of these results is complex because studies are heterogeneous, most of them also include malign and benign conditions, and different clinical situations (such as leaks, fistulas, stenosis or iatrogenic perforations) are analyzed together. In addition, different types of FCMS are included in these studies (Hanaro stent, ELLA stent, Alimaxx stent or Wallflex stent); for example, first reports about Wallflex dated from 2011 (30) and 2012 (31). Our results of initial and final success show great efficacy of our approach, similar to the best series published.
The choice of fully covered stents as treatment was subsequently endorsed by two comparative studies using different types of stents. Van Boeckel et al. (42) compared the efficacy of FCMS, PCMS and SEPS with slight increase of leak closure in the FCMS (83%) and plastic stents (83%) groups, versus 73% in the PCMS group. Main differences were found regarding migration rates (worse fully covered stents) and tissue in-or overgrowth (exclusively in partially covered stents). Gangloff et al. (43) reported greater long term clinical efficacy of FCMS (34.7%) versus PCMS (23.5%), without differences in migration rate. In a systematic review, stent migration occurred in 26% of patients with FCMS and 31% with SEPS (44).
Main concern about FCMS is the risk of migration, reported in up to 44% (27), although in most series it is about 20-30%. In order to reduce this problem, as we made previously (39), wider diameter stents were used to occlude completely the esophageal lumen obtaining a stronger anchor. Also, 48 hours in decubitus position was recommended to complete expansion of the stent. We cannot conclude that these two maneuvers are especially effective, but our migration rate is really low (13%), without complications related, and easy repositioning. Difficult in stent retrieval and severe tissue reactions were not found in our patients, in contrast to other series (31,33,36). Median of days between initial insertion of the stent and retrieval was 45, similar to other published series: Amrani et al., 5 weeks (27), Buscaglia et al., 42 days (30), Rajan et al., 53 days (36) or van Boeckel et al., 5-6 weeks (42). In our opinion, retrieval of the stent should not be performed before 4 weeks (ideally 6-8 weeks), in order to ensure closure of the leak, though it is a factor that does not seem to influence the risk of migration.
We found no difficulty in removal of stents, even in cases with periods of stent permanence longer than median. In the largest series published with FCMS (34) which included 59 patients (22 of them anastomotic leaks), stent-induced granulation tissue was described in 12 cases (20%). The mean duration of stent placement in these 12 cases was higher than in the median of all patients (88 versus 67 days), but all of them were successfully removed without complications.
According to our experience, the placement of FCMS is a good alternative in the treatment of postoperative leaks with minor risks, avoiding open reoperation in mostly of cases. Other potential include reducing the in-hospital stay and allowing early oral intakes. A low migration rate can be achieved, considering the size of the stent as one of the factors potentially involved. One advantage of our data is that we only describe, in contrast to most, post-surgical leaks in patients with esophageal or gastric cancer avoiding confounding etiologies. Major limitations are the absence of a control group, and being a single center study with a limited number of cases. Other main limitation is the fact that a single type of FCMS was used. A comparison between different types might be interesting and would provide more robust results.
In conclusion, the placement of FCMS to achieve the leak closure after esophageal or gastric surgery is an effective and probably, feasible without major complications, that could be considered in all cases.
References
1. Ikeguchi M, Oka S, Goymo Y, et al. Postoperative morbidity and mortality after gastrectomy for gastric carcinoma. Hepatogastroenterology 2001;48:1517-20. [ Links ]
2. Lang H. Piso P, Stukenborg C, et al. Management and results of proximal anastomotic leak in a serie of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol 2000;26:168-71. DOI: 10.1053/ejso.1999.0764. [ Links ]
3. Nowakowski P, Ziaja K, Ludyga T, et al. Self-expandable metallic stents in the treatment of post-esophagogastrostomy/post-esophagoenterostomy fistula. Dis Esophagus 2007;20:358-60. DOI: 10.1111/j.1442-2050.2007.00688.x. [ Links ]
4. Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophaguectomy for cancer: A systematic review and meta-analysis. Dig Surg 2011;28:29-35. DOI: 10.1159/000322014. [ Links ]
5. Saluja SS, Ray S, Pal S, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointestinal Surg 2012;16:1287-95 DOI: 10.1007/s11605-012-1885-7. [ Links ]
6. Briel JW, Tamhankar AP, Hagen L, et al. Prevalence and risk factors for ischemia, leakand stricture of esophageal anastomosis: Gastric pull-up versus colon interposition. J Am Coll Surg 2004;198:536-41. DOI: 10.1016/j.jamcollsurg.2003.11.026. [ Links ]
7. Rodella L, Laterza E, De Manzoni G, et al. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy 1998;30:453-6. DOI: 10.1055/s-2007-1001307. [ Links ]
8. García-Moreno JL, Suárez Grau JM, Gómez Bravo MA, et al. Closure of gastrocutaneous fistula using endoscopic biological glue injection. Rev Esp Enferm Dig 2007;99:676-7. [ Links ]
9. Evrard S, Le Moine O, Lazaraki G, et al. Self-expanding plastic stents for benign esophageal lesions. Gastrointest Endosc 2004;60:894-900. DOI: 10.1016/S0016-5107(04)02278-3. [ Links ]
10. Gelbmann CM, Ratiu NL, Rath HC, et al. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy 2004;36:695-9. DOI: 10.1055/s-2004-825656. [ Links ]
11. Hünerbein M, Stroszczynski C, Moesta KT, et al. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg 2004;240:801-7. DOI: 10.1097/01.sla.0000143122.76666.ae. [ Links ]
12. Schubert D, Scheidbach H, Kuhn R, et al. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc 2005;61:891-6. DOI: 10.1016/S0016-5107(05)00325-1. [ Links ]
13. Langer FB, Wenzl E, Prager G, et al. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg 2005;79:398-403. DOI: 10.1016/j.athoracsur.2004.07.006. [ Links ]
14. Radecke K, Gerken G, Treichel U. Impact of a self-expanding, plastic esophageal stent on various esophageal stenoses, fistulas, and leakages: A single-center experience in 39 patients. Gastrointest Endosc 2005;6:812-8. DOI: 10.1016/S0016-5107(05)00290-7. [ Links ]
15. Freeman RK, Ascioti AJ, Wozniak TC. Postoperative esophageal leak management with the Polyflex esophageal stent. J Thorac Cardiovasc Surg 2007;133:333-8. DOI: 10.1016/j.jtcvs.2006.10.008. [ Links ]
16. Al-Haddad M, Craig CA, Odell J, et al. The use of self-expandable plastic stents for non-malignant esophago-pleural fistulas. Dis Esophagus 2007;20:538-41. DOI: 10.1111/j.1442-2050.2007.00704.x. [ Links ]
17. Ott C, Ratiu N, Endlicher E, et al. Self-expanding Polyflex plastic stents in esophageal disease: Various indications, complications, and outcomes. Surg Endosc 2007;21:889-96. DOI: 10.1007/s00464-006-9067-x. [ Links ]
18. Pennathur A, Chang AC, McGrath KM, et al. Polyflex expandable stents in the treatment of esophageal disease: initial experience. Ann Thorac Surg 2008;85:1968-72. DOI: 10.1016/j.athoracsur.2008.01.095. [ Links ]
19. Leers JM, Vivaldi C, Schäfer H, et al. Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc 2009;23:2258-62. DOI: 10.1007/s00464-008-0302-5. [ Links ]
20. Tuebergen D, Rijcken E, Mennigen R, et al. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: Efficacy and current imitations. J Gastrointest Surg 2008;12:1168-76. DOI: 10.1007/s11605-008-0500-4. [ Links ]
21. Fischer A, Thomusch O, Benz S, et al. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg 2006;81:467-72. DOI: 10.1016/j.athoracsur.2005.08.047. [ Links ]
22. Johnsson E, Lundell L, Liedman B. Sealing of esophageal perforation or ruptures with expandable metallic stents: A prospective controlled study on treatment efficacy and limitations. Dis Esophagus 2005;18:262-6. DOI: 10.1111/j.1442-2050.2005.00476.x. [ Links ]
23. Wadhwa RP, Kozarek RA, France RE, et al. Use of self-expandable metallic stents in benign GI diseases. Gastrointest Endosc 2003;58:207-12. DOI: 10.1067/mge.2003.343. [ Links ]
24. Doniec JM, Schniewind B, Kahlke V, et al. Therapy of anastomotic leaks by means of covered self-expanding metallic stents after esophagogastrectomy. Endoscopy 2003;35:652-8. DOI: 10.1055/s-2003-41509. [ Links ]
25. Siersema PD, Homs MY, Haringsma J, et al. Use of large-diameter metallic stents to seal traumatic nonmalignant perforations of the esophagus. Gastrointest Endosc 2003;58:356-61. DOI: 10.1067/S0016-5107(03)00008-7. [ Links ]
26. Babor R, Talbot M, Tyndal A. Treatment of upper gastrointestinal leaks with a removable, covered, self-expanding metallic stent. Surg Laparosc Endosc Percutan Tech 2009;19:e1-4. DOI: 10.1097/SLE.0b013e318196c706. [ Links ]
27. Amrani L, Ménard C, Berdah S, et al. From iatrogenic digestive perforation to complete anastomotic disunion: endoscopic stenting as a new concept of "stent-guided regeneration and re-epithelialization". Gastrointest Endosc 2009;69:1282-7. DOI: 10.1016/j.gie.2008.09.043. [ Links ]
28. Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc 2009;23:1526-30. DOI: 10.1007/s00464-009-0432-4. [ Links ]
29. Han XW, Li YD, Wu G, et al. New covered mushroom-shaped metallic stent for managing anastomotic leak after esophagogastrostomy with a wide gastric tube. Ann Thorac Surg 2006;82:702-6. DOI: 10.1016/j.athoracsur.2006.02.078. [ Links ]
30. Buscaglia JM, Ho S, Sethi A, et al. Fully covered self-expandable metal stents for benign esophageal disease: a multicenter retrospective case series of 31 patients. Gastrointest Endosc 2011;74:207-11. DOI: 10.1016/j.gie.2011.02.024. [ Links ]
31. Wagh MS, Forsmark CE, Chauhan S, et al. Efficacy and safety of a fully covered esophageal stent: a prospective study. Gastrointest Endosc 2012;75:678-82. DOI: 10.1016/j.gie.2011.10.006. [ Links ]
32. Senousy BE, Gupte AR, Draganov PV, et al. Fully covered Alimaxx esophageal metal stents in the endoscopic treatment of benign esophageal diseases. Dig Dis Sci 2010;55:3399-403. DOI: 10.1007/s10620-010-1415-y. [ Links ]
33. Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol 2009;104:1374-81. DOI: 10.1038/ajg.2009.133. [ Links ]
34. Bakken JC, Wong Kee Song LM, de Groen PC, et al. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest Endosc 2010;72:712-20. DOI: 10.1016/j.gie.2010.06.028. [ Links ]
35. Wilson JL, Louie BE, Farivar AS, et al. Fully covered self-expanding metal stents are effective for benign esophagogastric disruptions and strictures. J Gastrointest Surg 2013;17:2045-50. DOI: 10.1007/s11605-013-2357-4. [ Links ]
36. Rajan PS, Bansal S, Balaji NS, et al. Role of endoscopic stents and selective minimal access drainage in oesophageal leaks: Feasibility and outcome. Surg Endosc 2014;28:2368-73. DOI: 10.1007/s00464-014-3471-4. [ Links ]
37. Sharaiha RZ, Kim KJ, Singh VK, et al. Endoscopic stenting for benign upper gastrointestinal strictures and leaks. Surg Endosc 2014;28:178-84. DOI: 10.1007/s00464-013-3150-x. [ Links ]
38. Roy-Choudhury SH, Nicholson AA, Wedgwood KR, et al. Symptomatic malignant gastroesophageal anastomotic leak: Management with covered metallic esophageal stents. AJR Am J Roentgenol. 2001;176:161-5. DOI: 10.2214/ajr.176.1.1760161. [ Links ]
39. Fernandez A, Vila JJ, Vazquez S, et al. Self-expanding plastic stent for the treatment of post-operative esophago-jejuno anastomosis leak. A case series study. Rev Esp Enferm Dig 2010;102:704-10. DOI: 10.4321/S1130-01082010001200005. [ Links ]
40. Hirdes MM, Vleggaar FP, Van der Linde K, et al. Esophageal perforation due to removal of partially covered self-expanding metal stents placed for a benign perforation or leak. Endoscopy 2011;43:156-9. DOI: 10.1055/s-0030-1255849. [ Links ]
41. Hirdes MM, Siersema PD, Houben MH, et al. Stent-in-stent technique for removal of embedded esophageal self-expanding metal stents. Am J Gastroenterol 2011;106:286-93. DOI: 10.1038/ajg.2010.394. [ Links ]
42. van Boeckel PG, Dua KS, Weusten BL, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol 2012;29;12:19. DOI: 10.1186/1471-230X-12-19. [ Links ]
43. Gangloff A, Lecleire S, Di Fiore A, et al. Fully versus partially covered self-expandable metal stents in benign esophageal strictures. Dis Esophagus 2014 Aug 29. doi: 10.1111/dote.12260. DOI: 10.1111/dote.12260. [ Links ]
44. Van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: Temporary stent placement for benign rupture or anastomotic leak of the esophagus. Aliment Pharmacol Ther 2011;33:1292-301. DOI: 10.1111/j.1365-2036.2011.04663.x. [ Links ]
![]() Correspondence:
Correspondence:
Alberto Fernández Villaverde.
Department of Digestive Diseases.
Hospital POVISA.
C/ Salamanca, 5. 36211 Vigo,
Pontevedra. Spain
e-mail: afvillaverde@gmail.com
Received: 13-03-2015
Accepted: 02-06-2015