My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 n.2 Madrid Feb. 2016
ORIGINAL PAPERS
Predictive factors of small bowel patency in Crohn's disease patients
Andreia Albuquerque1, Hélder Cardoso1, Margarida Marques1, Susana Rodrigues1, Filipe Vilas Boas1, Susana Lopes1, Cláudia Camila Dias2 and Guilherme Macedo1
1Gastroenterology Department. Centro Hospitalar São João. Porto, Portugal.
2CIDES - Department of Health Information and Decision Sciences. Faculty of Medicine. University of Porto. Portugal
ABSTRACT
Background: Patency capsule was developed to avoid small bowel video capsule endoscopy retention, namely in patients with Crohn's disease.
Aims: To evaluate the predictive factors of small bowel patency in Crohn's disease patients.
Patients and methods: Retrospective analysis including 151 Crohn's disease patients submitted to patency capsule (Agile® Patency Capsule) from 2011 to 2012. Patients that excreted the intact patency capsule were classified as having a patent small bowel (without patency capsule retention), other patients were considered to have negative patency of the small bowel (patency capsule retention).
Results: Patients had a mean age of 41±14 years, 54% were female and 25% had been previously submitted to surgery. Stricturing disease was seen in 20% of cases and penetrating disease in 16% of cases. Left-sided colonic lesions and ileal strictures were observed at colonoscopy in 13% and 9% of patients, respectively. In our sample, 28% of patients had negative patency of the small bowel (patency capsule retention). In multivariate analysis, independent factors that were associated with negative patency of the small bowel in Crohn's disease patients were stricturing (OR 10.16, p < 0.001) and penetrating phenotypes (OR 11.73, p = 0.001), left-sided colonic lesions (OR 3.77, p = 0.038), ileal stricture (OR 9.76, p = 0.003); previous intestinal surgery was found to be protective (OR 0.16, p = 0.006).
Conclusions: Stricturing or penetrating disease, ileal strictures, no previous surgery and left-sided colonic lesions were the factors associated with negative small bowel patency in Crohn's disease patients.
Key words: Crohn's disease. Patency capsule. Small bowel video capsule endoscopy. Small bowel patency.
Introduction
In 2005, patency capsule (PC) was developed to avoid small bowel video capsule endoscopy (SBVCE) retention, namely in patients with Crohn's disease (CD), providing direct evidence of functional patency of the gut lumen (1-5). SBVCE cannot be recommended as a safe procedure in patients in whom the PC disintegrates or if pain is experienced during its passage (1).
Capsule retention is defined as having a videocapsule remaining in the digestive tract for a minimum of two weeks and requiring directed medical, endoscopic, or surgical intervention for removal (6). The rate of retention in patients with obscure gastrointestinal bleeding is 1.5% (7), in patients with known CD the rate of retention is 13%, and in patients with suspected CD, the retention rate is 1.6% (8). The European evidence based consensus for endoscopy in inflammatory bowel disease (IBD) recommend that in patients with established CD, cross sectional imaging or PC should be performed when SBVCE is being considered, in order to identify stenosis that may cause capsule retention (9). One retrospective study compared the performance of the PC and radiological examinations to detect clinically significant small bowel (SB) strictures and both methods were equivalent (10) but, there are other studies that reported that radiological test have higher false positive rate compared with PC test (11,12). In a multicenter study of 106 patients with evidence of intestinal strictures on Computed Tomography (CT) or small bowel follow-through, 56% had a negative PC test results and subsequently underwent uneventful SBVCE (11). Radiological techniques have several others limitations, namely, high radiation dose, lack of precise definition of the functional feature of the stricture, and can also have false negative results, especially when obstruction is intermittent or partial (13). PC on the other hand, it is a simple minimally invasive outpatient procedure, requiring no bowel preparation (10).
It is important to know what group of CD patients would be at a higher risk of retention, beyond the presence of SB strictures, so that it would be possible to have a systematic approach for diagnostic workup of CD patients before SBVCE.
The aim of this study was to evaluate the predictive factors of SB patency in CD patients, namely which patients have a higher risk of negative SB patency and therefore should always be submitted to PC before SBVCE.
Patients and methods
This was a retrospective analysis that included adult patients with the diagnosis of CD (≥ 18 years) submitted to PC (Agile® Patency Capsule, Given Imaging Ltd, Yoqneam, Israel) in a two-year period, from January 2011 to December 2012.
During this time, 151 PC were performed in CD patients, one PC per patient. Patients gave previous written informed consent to the procedure.
Agile® PC is 26 mm long, 11 mm wide device, composed of lactose, 5% barium sulfate, a small inner radiofrequency identification tag (RFIT) that allows scanner identification, two timer plugs that seal the capsule's body, and an impermeable membrane (except for a small window in the timer plug). Fluid in the GI tract causes slow erosion of the timer plug, allowing the penetration into the capsule and its disintegration. The system includes a radiofrequency identification scanner to allow detection of the tag in situ.
Agile® capsule, was developed in order to minimize the risk of occlusion in comparison with the Given® M2A Patency System (14). Agile® Capsule starts its dissolution process earlier (30 h after ingestion) and its two-timer plugs have been designed to begin the disintegration even when the device is blocked in a tight stricture (5).
After a 12-hour fasting period, PC was ingested. Scanner detection and symptoms evaluation were assessed 30 hours after PC ingestion. The intestinal tract was considered to be patent (without PC retention) if the capsule was excreted intact, or if the capsule was not detected by the scanner at 30h after ingestion. The excreted PC was considered intact when no changes were observed in the dimension and solidity of the body capsule. All these patients were then submitted to SBVCE.
Patients that had positive scanner detection were submitted to an abdominal X-ray to identify PC localization. None of these patients underwent SBVCE.
All of the patients had a previous colonoscopy performed. For the analysis we considered patients with isolated lesions in the descending or sigmoid colon and those with involvement of both segments (left-sided colonic lesions). Colonoscopy lesions were defined by the presence of erosions and/or ulcers. Ileal stricture was defined by the presence of a stricture in the ileum seen in the ileocolonoscopy, with the inability to intubate the terminal ileum.
Montreal classification prior to SBVCE was used for CD classification.
Anemia was defined as hemoglobin level < 12 g/dL in woman and < 13 g/dL in men.
There were several characteristics that were evaluated as possible predictors of SB patency, namely, gender, age at PC, CD disease duration, previous IBD surgery, CD classification (Montreal), CD treatment, colonic lesions at colonoscopy, anemia and C-reactive protein levels.
Statistical analysis was performed with version 21 of SPSS software (SPSS Inc, Chicago, USA). A P value of less than 0.05 was considered significant. Data was analyzed using Chi-Square test for categorical variables, independent-samples T-test, and Mann-Whitney U nonparametric test for continuous variables and logistic regression for multivariate analysis.
Results
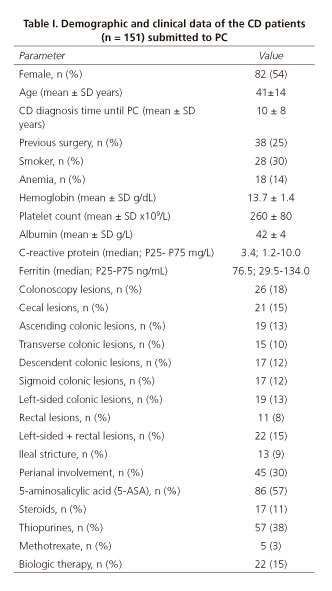
Patients submitted to PC were female in 54% of the cases, with a mean age of 44±14 years, a mean CD diagnosis time until PC of 10 ± 8 years (Table I).
In patients with CD diagnosis the major indication for SBVCE was SB evaluation; other indications were anemia, diarrhea, increased inflammatory biomarkers, and post-operative evaluation. Fourteen percent of the patients (n = 18) had anemia, only 4 of these patients had a negative SB patency test. Regarding CD classification (Montreal classification), most of the patients had been diagnosed between 17-40 years old (A2 69%), had non-stricturing, non-penetrating behavior (B1 64%), and ileal location (L1 58%) (Table II). There was perianal involvement in 30% of the cases. Concerning CD treatment, 57% of the patients were on 5-aminosalicylic acid (5-ASA), 11% on steroids, 32% on thiopurines, 3% on methotrexate, and 12% on biologics. All of the patients had a previous colonoscopy, and lesions were detected: 14% in the cecum, 13% in the ascending colon, 10% in the transverse colon, 13% in the left-sided colon, and 11% in the rectum. Thirteen patients (9%) had an ileal stricture documented in the colonoscopy and 10 of these patients had PC retention.
In our series, SB patency was negative in 28% (n = 42) of the cases and there were no severe adverse events in these patients, namely obstruction or hospital admission. In the 72% of cases the SB was patent, and these patients were submitted to SBVCE, with no capsule retention.
Negative SB patency was associated with stricturing and penetrating CD phenotypes (p < 0.001), with left-sided colonic lesions (p = 0.047) and ileal strictures (p < 0.001) (Table III).
The variables with significant association by univariate analysis were selected for the regression model. The variables independently associated with negative SB patency in CD patients, using logistic regression, were stricturing (OR 10.16; 95% CI 3.12 to 33.04; p < 0.001) and penetrating CD phenotypes (OR 11.73; 95% CI 2.78 to 49.44; p = 0.001), left-sided colonic lesions (OR 3.77; 95% CI 1.08 to 13.19; p = 0.038), and ileal strictures (OR 9.76; 95% CI 2.19 to 43.50; p = 0.003), while previous intestinal surgery was found to be protective (OR 0.16; 95% CI 0.04 to 0.60; p = 0.006) (Table IV). The receiver operating characteristic (ROC) curve showed an area under the curve (AUC) of 0.806 (95% CI 0.718-0.894) (Fig. 1).
Among the 19 patients with left-sided colonic lesions, 9 had negative SB patency. In total, 38 patients (25%) had been previously submitted to surgery and, of these, 10 patients had negative SB patency (PC detection/retention). When we analyzed this results considering CD behavior, of the 97 patients with non-stricturing, non-penetrating behavior, 9 patients had been previously submitted to surgery and none of these patients had PC detection (p = 0.34).Regarding patients with stricturing and penetrating CD phenotypes (n = 54), 29 patients had been previously submitted to surgery and 10 of these had PC detection (p = 0.028).
Discussion
In this study, 72% of the patients submitted to PC had SB patency and could undergo SBVCE. Others previous series had a similar rate of patients with SB patency, like in the serie of Boivin et al. (1) (73%), Delvaux et al. (14) (73%) and Signorelli et al. (2) (81%). All of the patients with a patent SB were submitted to SBVCE and there were no cases of capsule retention. In our department, PC is always performed previously to SBVCE in patients with diagnosed CD. We currently have over 2,000 patients in our IBD clinic.
The cumulative rates of complications in patients with CD have been reported to range from 48-52% at 5 years and 69-70% at 10 years after diagnosis, with approximately half of the patients developing a stricture (15-17) most commonly in the ileum or ileo-cecal region (18,19). In total, 13 patients with ileal strictures documented by colonoscopy were submitted to PC, and 3 of these patients had a patent SB, despite the documented ileal stricture. Patients with a bowel stricture, but with PC passage, can undergo SBVCE safely, reinforcing the advantage of always performing PC in these patients. Nevertheless, logistic regression analysis demonstrated that ileal stricture is independently associated with negative SB patency in CD patients (OR 9.76, p = 0.003).
Both stricturing (OR 10.16, p < 0.001) and penetrating CD phenotypes (OR 11.73, p = 0.001) were independently associated with negative SB patency in CD patients, using logistic regression. In 2010, Jurgens et al. (20) published a study showing that penetrating CD was strongly associated with concomitant intestinal strictures, and the presence of fistulas resulted in a positive predictive value of 86.2% for predicting intestinal strictures. Fistulas are thought to develop in regions of full thickness bowel wall inflammation in a high-pressure region upstream from a stricture (21,22).
Left-sided colonic involvement (OR 3.77, p = 0.038) was also independently associated with a negative SB patency in patients with CD diagnosis. No differences were found when considering only descending or sigmoid colonic lesions as a predictive factor for SB patency. Among the 19 patients with left-sided colonic lesions, 9 had negative SB patency. There was no link between these lesions and ileal strictures (p = 0.644), only one patient with left-sided colonic lesions had ileal strictures. As far as we know, left-sided colonic involvement was never previously found to be a predictive factor of negative SB patency in CD diagnosis. In our Department we have conducted a study that show that sigmoid lesions seen at colonoscopy were predictive of proximal SB involvement at SBVCE (presented as a poster at DDW 2014: Rodrigues S, Cardoso H, Rosa B, et al. Sa1218 Predictive factors of proximal small bowel Crohn's disease detected at capsule endoscopy. Gastroenterology, Vol. 146, issue 5, S-233). Our hypothesis is that patients with left-colonic lesions could have more proximal SB involvement and, due to this, a higher risk of retention. We could not determine this in our study because patients with negative SB patency were not submitted to SBVCE. Sigmoid lesions may not have been a predictive factor of SB patency in this study due to the small number of patients with these lesions. This data can be very important and needs to be confirmed in future studies.
In the absence of treatment, the post-operative recurrence rate is around 65-90% within 12 months and 80-100% within 3 years of the operation (23). The development of an early severe endoscopic recurrence within one year represents a risk factor for clinical recurrence and timely detection may allow for appropriate treatment of CD patients after surgery. Ileocolonoscopy currently represents the gold standard for assessing CD recurrence, graded according to the Rutgeerts' score. Several alternative, noninvasive techniques have been used in order to assess the post-operative recurrence, including: fecal alpha 1-antitrypsin clearance, fecal calprotectin, 99Tc-HMPAO scintigraphy, virtual colonoscopy, ultrasonography, and SBVCE (24-26). CD patients previously submitted to intestinal surgery were found to have less PC detection/retention (OR 0.16, p = 0.006). Amongst the 38 patients with intestinal surgery in our serie, stricturing and penetrating disease was the main indication for surgery (in 29 of the 38 patients). All of the 10 patients with previous surgery that had PC retention had a stricturing or penetrating behavior, and most were submitted to PC less than a year after surgery. This might explain how previous intestinal surgery was a protective factor.
The ROC curve of the regression model showed an AUC of 0.806, consistent with a good predictive performance for SB patency in CD.
This is a large study involving CD submitted to of PC and was performed in a single center, following the same PC protocol. One of the limitations of the PC examination is the false-positive result that can occur when the RFIT is in the colon rather than the SB, thereby precluding SBVCE (17). We used abdominal radiography to localize the RFIT identified 30 h after the ingestion. CT examination is a better method, but is not routinely performed or indicated after PC positivity, mainly because of the radiation and cost-effective. Also, our PC retention rate was similar to those previously describe in others series.
Due to high retention rate of SBVCE in CD patients (13%), it is recommended that those with established CD have a cross sectional imaging or PC performed when SBVCE is being considered. However, these methods have a cost and limitations and it is important to know which CD patients are at a higher risk for retention. Our study evaluated the predictive factors of SB patency, some of them were not previously reported. We showed that the risk of capsule retention is not only related with the presence of strictures or stricturing phenotype. Penetrating disease, no previous surgery and left-sided colonic lesions were also factors associated with negative SB patency in CD patients. Although more studies are needed, the presence of these characteristics should alert clinicians for a higher risk of retention. This can allow for an improved selection of CD patients for PC and SBVCE, in order to avoid retention.
References
1. Boivin ML, Lochs H, Voderholzer WA. Does passage of a patency capsule indicate small-bowel patency? A prospective clinical trial. Endoscopy 2005;37:808-15. DOI: 10.1055/s-2005-870220. [ Links ]
2. Signorelli C, Rondonotti E, Villa F, et al. Use of the Given Patency System for the screening of patients at high risk for capsule retention. Dig Liver Dis 2006;38:326-30. DOI: 10.1016/j.dld.2006.01.010. [ Links ]
3. Spada C, Spera G, Riccioni M, et al. A novel diagnostic tool for detecting functional patency of the small bowel: The Given patency capsule. Endoscopy 2005;37:793-800. DOI: 10.1055/s-2005-870246. [ Links ]
4. Spada C, Riccioni ME, Costamagna G. The new, dissolving patency capsule: A safe and effective tool to avoid the complication of retained video capsules. J Clin Gastroenterol 2008;42:761-2. DOI: 10.1097/MCG.0b013e31802e7f11. [ Links ]
5. Caunedo-Alvarez A, Romero-Vazquez J, Herrerias-Gutierrez JM. Patency and Agile capsules. World J Gastroenterol 2008;14:5269-73. DOI: 10.3748/wjg.14.5269. [ Links ]
6. Cave D, Legnani P, de Franchis R, et al.; ICCE. ICCE consensus for capsule retention. Endoscopy 2005;37:1065-7. DOI: 10.1055/s-2005-870264. [ Links ]
7. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis 2010;4:7-27. DOI: 10.1016/j.crohns.2009.12.003. [ Links ]
8. Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol 2006;101:2218-22. DOI: 10.1111/j.1572-0241.2006.00761.x. [ Links ]
9. Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013;7:982-1018. DOI: 10.1016/j.crohns.2013.09.016. [ Links ]
10. Yadav A, Heigh RI, Hara AK, et al. Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc 2011;74:834-9. DOI: 10.1016/j.gie.2011.05.038. [ Links ]
11. Herrerias JM, Leighton JA, Costamagna G, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc 2008;67:902-9. DOI: 10.1016/j.gie.2007.10.063. [ Links ]
12. Postgate AJ, Burling D, Gupta A, et al. Safety, reliability, and limitations of the Given patency capsule in patients at risk of capsule retention: A 3-year technical review. Dig Dis Sci 2008;53:2732-8. DOI: 10.1007/s10620-008-0210-5. [ Links ]
13. Spada C, Shah SK, Riccioni ME, et al. Video capsule endoscopy in patients with known or suspected small bowel stricture previously tested with the dissolving patency capsule. J Clin Gastroenterol 2007;41:576-82. DOI: 10.1097/01.mcg.0000225633.14663.64. [ Links ]
14. Delvaux M, Ben Soussan E, Laurent V, et al. Clinical evaluation of the use of the M2A patency capsule system before a capsule endoscopy procedure, in patients with known or suspected intestinal stenosis. Endoscopy 2005;37:801-7. DOI: 10.1055/s-2005-870241. [ Links ]
15. Rieder F, Zimmermann EM, Remzi FH, et al. Crohn's disease complicated by strictures: A systematic review. Gut 2013;62:1072-84. DOI: 10.1136/gutjnl-2012-304353. [ Links ]
16. Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001;49:777-82. DOI: 10.1136/gut.49.6.777. [ Links ]
17. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 2002;8:244-50. DOI: 10.1097/00054725-200207000-00002. [ Links ]
18. Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: Report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 2000;6:8-15. DOI: 10.1097/00054725-200002000-00002. [ Links ]
19. Fukumoto A, Tanaka S, Yamamoto H, et al. Diagnosis and treatment of small-bowel stricture by double balloon endoscopy. Gastrointest Endosc 2007;66:S108-12. DOI: 10.1016/j.gie.2007.02.027. [ Links ]
20. Jürgens M, Brand S, Laubender RP, et al. The presence of fistulas and NOD2 homozygosity strongly predict intestinal stenosis in Crohn's disease independent of the IL23R genotype. J Gastroenterol 2010;45:721-31. DOI: 10.1007/s00535-010-0231-7. [ Links ]
21. Oberhuber G, Stangl PC, Vogelsang H, et al. Significant association of strictures and internal fistula formation in Crohn's disease. Virchows Arch 2000;437:293-7. DOI: 10.1007/s004280000226. [ Links ]
22. Sheehan AL, Warren BF, Gear MW, et al. Fat-wrapping in Crohn's disease: Pathological basis and relevance to surgical practice. Br J Surg 1992;79:955-8. DOI: 10.1002/bjs.1800790934. [ Links ]
23. Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Special situations. J Crohns Colitis 2010;4:63-101. DOI: 10.1016/j.crohns.2009.09.009. [ Links ]
24. Ciorba MA, Prakash C. Wireless capsule endoscopy in the diagnosis of small bowel Crohn's disease. Inflamm Bowel Dis 2003;9:276. DOI: 10.1097/00054725-200307000-00010. [ Links ]
25. Biancone L, Onali S, Calabrese E, et al. Non-invasive techniques for assessing postoperative recurrence in Crohn's disease. Dig Liver Dis 2008;40(Supl. 2):S265-70. DOI: 10.1016/S1590-8658(08)60536-8. [ Links ]
26. Buisson A, Chevaux JB, Bommelaer G, et al. Diagnosis, prevention and treatment of postoperative Crohn's disease recurrence. Dig Liver Dis 2012;44:453-60. DOI: 10.1016/j.dld.2011.12.018. [ Links ]
![]() Correspondence:
Correspondence:
Andreia Albuquerque.
Gastroenterology Department.
Centro Hospitalar São João.
Alameda Professor Hernâni Monteiro.
4200-319 Porto, Portugal
e-mail: a.albuquerque.dias@gmail.com
Received: 05-08-2015
Accepted: 30-09-2015