Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.6 Madrid jun. 2016
Flexible endoscopic treatment of Zenker's diverticulum: thirteen years' experience in Spain
Tratamiento endoscópico flexible del divertículo de Zenker: trece años de experiencia en España
Emilio J. de-la-Morena-Madrigal1,2, Elena Pérez-Arellano1 and Isabel Rodríguez-García1
1 Department of Digestive Diseases. Hospital La Zarzuela. Madrid, Spain.
2 DMQ. Hospital Beata María Ana de Jesús. Madrid, Spain
ABSTRACT
Introduction: Flexible endoscopic treatment is one of the alternative approaches for the management of Zenker's diverticum. The present paper shows our short-term and long-term results with flexible endoscopic cricopharyngeal myotomy/septotomy.
Patients and methods: A retrospective analysis of our experience in patients with Zenker's diverticulum treated using a flexible endoscope, assisted by a flexible diverticuloscope, between 2002 and 2015. Myotomy/septotomy was performed with a needle-knife papillotome under deep sedation or general anesthesia.
Results: Among the 64 patients treated, two died within 10 days of surgery from causes not directly related to the procedure, and one presented with pharyngo-esophageal perforation, which recovered with conservative management at 47 days after admission. Four additional patients were lost to short-term follow-up. Among the 57 remaining patients, 52 had complete relief of dysphagia after 6 weeks. Eleven of these had recurrent symptoms on the mid and the long term. Eight were retreated with the same flexible endoscopic technique, one with a hybrid endoscopic approach, one with classical open surgery and one refused retreatment. After a mean follow-up of 2 years and a half, 33 of 37 patients reported absent or minimal dysphagia, controllable with punctual dietary restrictions.
Conclusions: Flexible endoscopic treatment for Zenker's diverticulum is effective and safe. It represents an option on an equal footing to rigid endoscopy and classical open surgery and may also be used when the latter two are technically impracticable or contraindicated.
Key words: Zenker's diverticulum. Flexible endoscopic treatment. Cricopharyngeus myotomy/septotomy.
RESUMEN
Introducción: el tratamiento endoscópico flexible del divertículo de Zenker es una alternativa a otros abordajes terapéuticos. El presente estudio muestra nuestros resultados a corto y largo plazo de la crico-faringo-mío-septotomía endoscópica flexible.
Pacientes y método: análisis retrospectivo de nuestra experiencia entre 2002 y 2015 en pacientes con divertículo de Zenker tratados mediante un endoscopio flexible con la asistencia de un diverticuloscopio flexible. La mío-septotomía se realiza con un papilotomo de aguja bajo sedación profunda o anestesia general.
Resultados: de los 64 pacientes tratados dos fallecieron antes de 10 días después del procedimiento por causas no directamente relacionadas con la intervención y otro presentó una perforación faringo-esofágica que se resolvió con tratamiento conservador tras 47 días de ingreso. Carecemos de seguimiento a corto plazo de otros cuatro. Cincuenta y dos de los 57 restantes mostraron un alivio completo de la disfagia a las 6 semanas. Once de ellos presentaron recurrencia sintomática a medio o largo plazo. Ocho fueron retratados con el mismo método endoscópico flexible, uno mediante un abordaje endoscópico híbrido, otro mediante cirugía abierta clásica y otro rechazó el retratamiento. Tras un seguimiento medio de 2 años y medio, 33 de 37 pacientes refieren ausencia o mínima disfagia controlable con restricciones dietéticas puntuales.
Conclusiones: el tratamiento endoscópico flexible del divertículo de Zenker es eficaz y seguro. Representa una alternativa en igualdad de condiciones al abordaje endoscópico rígido y a la cirugía abierta clásica y puede aplicarse cuando existe imposibilidad técnica o contraindicación para estos.
Palabras clave: Divertículo de Zenker. Tratamiento endoscópico flexible. Crico-faringo-mío-septotomía.
Introduction
Zenker's diverticulum (ZD) is a false diverticulum at the pharyngoesophageal junction, located above the upper esophageal sphincter, that results from dysfunction of the cricopharyngeal muscle, which loses its ability to relax during deglutition. The consequent increase in hypopharyngeal pressure results in herniation of the pharyngeal mucosal and submucosal layers through the weak spot entailed by Killian's dehiscence, on the posterior hypopharyngeal wall between the thyropharyngeus and cricopharyngeus muscles. The adventitial layers of the diverticular wall and the esophageal wall merge together into a solid septum (with no virtual cavity in between) separating both lumina.
Although not universally consistent in early clinical stages, oropharyngeal dysphagia, which results from impaired cricopharyngeal muscle relaxation, is the primary symptom of this disorder and may become severe even for small-size diverticula, particularly when associated with other motor swallowing disorders. Food retention with risk for bronchoaspiration develops as the diverticulum progressively grows. Upon reaching a threshold size, the diverticulum and its contents compress the cervical esophagus, thus adding a new pathophysiological factor to dysphagia. Malnutrition predominates in advanced stages, and compromises life prognosis should complications arise.
Throughout the 20th century, the outcomes of the various surgical techniques in use showed the crucial role of cricopharyngeus myotomy, regardless of diverticular excision, for therapy success (1,2). From the 1960s on, rigid endoscopic cricopharyngeus myotomy/septotomy (CPMST) proved effective (3), and the technique became popular and widespread among surgeons and ENT specialists during the 1990s because of endostaplers (4). Also during the 1990s papers started reporting the feasibility and efficacy of flexible endoscopic CPMST (5-8). The technical variants developed along the 20 years elapsed since then, still pending standardization and direct comparison, have shown efficacy and safety to the point of considering flexible endoscopy a same-level alternative to rigid endoscopy rather than merely a rescue option (9).
The goal of the present paper is to discuss our experience and the lessons learned from flexible endoscopic treatment (FET) for ZD along 13 years.
Patients and Method
We performed a retrospective analysis of all ZD patients treated by the first author in several hospitals. Sources for the analysis include patient medical records, both paper-based and electronic, and the available video recordings of endoscopic procedures. Data relating to clinical and imaging manifestations before treatment, surgical technique, outcome, early complications, and long-term follow-up, including need for retreatment and cause of death, when applicable, were recorded.
In addition to the general approach, we performed a comparative analysis of variables (sedation type, papillotome type, endoscope type) potentially determining a negative result (defined as any serious complication directly associated with the procedure and/or retreatment indication).
Dysphagia was categorized into five levels: no dysphagia (level 0), solid dysphagia (level 1), semisolid dysphagia (level 2), liquid dysphagia (level 3), and total aphagia (level 4) (10).
Pentax (Pentax Co., Tokyo, Japan), Fujinon (Fujifilm Co., Tokyo, Japan), and Olympus (Olympus Medical Systems, Tokyo, Japan) gastroscopes were used, with calibers ranging from 8.5 to 9.8 mm and working channels ranging from 2.8 to 3.2 mm in diameter. Pentax and Fujinon gastroscopes (PFG) are considered as equivalent for the subgroup analysis, since their working channels emerge at the 5-6 o'clock position in the visual field, whereas the 8-9 o'clock position is used by Olympus gastroscopes (OG).
Procedure
All patients signed an informed consent and received antibiotic prophylaxis with 1 g IV amoxicillin-clavulanic acid. Depending on each site, deep sedation was administered by an anesthesiologist or a nurse supervised by the endoscopist, or general anesthesia was provided by an anesthesiologist.
The flexible diverticuloscope (DSC) ZDO-22-30 (Cook Medical, Bloomington IN, USA), preset over the endoscope's tube (with its end inside the gastric antrum), is introduced with its short flap in the palatal position and the long flap in the lingual position. When the circular mark on the DSC surpasses the incisive line its location is checked, and corrected when applicable, under endoscopic view. Should this method fail, a 0,035" guidewire is passed into the stomach, the endoscope is withdrawn, and a channel is opened in DSC's long flap using an 18G IV catheter, through which the guidewire is passed to implant the DSC without using the endoscope.
CPMST was performed using a needle-knife papillotome (Cook Medical. Bloomington IN, USA) of the Huibregtse type (HPC-2) during 2002-2007, and of the Zimmon type (PTW-1) during 2008-2015. In over 95% of cases a diathermy generator ERBE ICC-200 (ERBE Elektromedizin, Tübingen, Germany) was used to apply a mixed current with cutting power restricted to 80 W, coagulation set at level 3, and "endocut" mode activated. Sections were performed in the ventrodorsal direction, and extended to resect ⅔-¾ of the septum, leaving a safety margin of 5 to 10 mm. In case of bleeding during CPMST with a Huibregtse papillotome (HP), argon plasma coagulation (APC) was applied using an ERBE APC-2 (ERBE Elektromedizin, Tübingen, Germany) generator. For CPFMST using a Zimmon papillotome (ZP) either CPA was also used or, for minimum bleeding, monopolar electrocoagulation (MPEC) with the ZP, its coagulation current set in the forced mode and power limited to 60 W, was aplied. During the 2002-2012 period no hemostatic clips were implanted at the bottom of the incision to prevent delayed perforation, but this was consistently carried out during the last three years using Resolution (Boston Scientific. Natick MA, USA) clips.
Once the DSC was withdrawn the neck was manually examined to rule out subcutaneous emphysema. Neck and/or chest x-rays were only used for suspected perforation. In the latter's absence, patients initiated a liquid diet at 12-18 hours with no prior radiographic assessment using a water-soluble contrast medium. Patients were discharged at 36-48 hours, and their first clinical and endoscopic follow-up was scheduled after 4 to 8 weeks.
Statistical analysis
The statistical analysis was accomplished using the SPSS 15.0 (Lead Technologies, Chicago ILL, USA) package. Descriptive data were expressed as mean (range) values for continuous variables. Dysphagia grade was considered as a continuous variable, and was expressed as mean ± SD. Pearson's χ2 test was used to test independence between categorical variables, and Student's t-test was used for mean comparisons between quantitative variables. Statistical significance was set at p < 0.05.
Results
During the period from May 2002 to June 2015, 64 patients (males 72%) with a mean age of 71.8 (41-96) years received treatment. Four had a family history of ZD: two of them were a mother and her son, and two had siblings (one was a twin) with ZD. Two patients had undergone surgery of the cervical spine 20 and 12 years, respectively, before their CPMST; one had had a retro-sternocleidomastoid branchigenic cyst excised 33 years before, and one had received repair surgery for a left brachial plexus injury following a traffic accident 27 years before. One patient had undergone surgical diverticulectomy 5 years before and one a first flexible endoscopic procedure in a different site 10 years ago.
Initial dysphagia grade was 1.45 ± 0.89, with the distribution shown in table I. Five patients did not report dysphagia, and one female patient reported total aphagia from acute diverticulitis. Eight had suffered at least one aspiration pneumonia event and five exhibited severe malnutrition.
ZD size, as estimated with endoscopy, was 3.2 (1.5-6) cm. Thirteen patients had food remnants inside their diverticulum despite their having fasted for at least 8 hours, four had a pill, whole or crumbled, and two exhibited intradiverticular candidiasis.
The first three cases (previously reported) were managed before the arrival of flexible DSC, with two orogastric tubes being used to assist CPMST (11). The remaining 61 patients were managed with a flexible DSC. Four patients, one of them with drug-related diverticulitis, required guidewire assitance for implantation.
Early incidents and complications
Twenty-one patients (33%) had incidental bleeding during the procedure, which was controlled with APC in 17 and MPEC with ZP in four. A patient with pulsating bleeding received one clip after APC. No patients had subcutaneous emphysema after APC. In two patients CPMST was incomplete because of visual interference by blood remnants. In two additional patients CPMST was incomplete because of poor tolerability; one was under sedation by an anesthesiologist, the other under sedation by the endoscopist.
Two patients presented with moderate chest pain (one also with fever) and one patient had fever without chest pain. Chest X-rays were normal for all three cases, which were discharged after 48 hours.
Dehiscence at the esophageal and diverticular walls upon DSC withdrawal was seen in one patient (Fig. 1A), who received two clips (Fig. 1B) and was discharged after 48 hours.
An 82-year-old woman had subcutaneous emphysema 24 hours after the procedure, which was massive (Fig. 2A) and associated with pneumomediastinum on the CT scan. An endoscopy procedure at 11 days showed a large perforation at the pharyngo-esophageal junction (Fig. 2B). This was managed conservatively with antibiotics and a radiographically guided gastrostomy, with the patient being discharged in good health and with no dysphagia 47 days after admission. A review of the procedure recordings showed that on DSC withdrawal CPMST was virtually complete, though no esophageal-diverticular wall dehiscence was apparent. Following this case (no. 38) routine clip implantation at the bottom of the incision was added to the protocol.
Two patients with severe wasting, an 84-year-old male and an 81-year-old woman with advanced Parkinson's disease and dementia, died at days 9 and 7, respectively, after CPMST, the former from a perforated duodenal ulcer, the latter from bronchoaspiration.
Median hospital stay, excluding the above two early demises and the esophageal perforation case, was 2 (1-6) days.
Follow-up and retreatment
The above two deaths and esophageal perforation case aside, 4 patients were lost of short-term follow-up. In the 57 patients reviewed at 54 (8-118) days, complete clinical response was above 90% and dysphagia grade had fallen to 0.04 ± 0.2, with the distribution shown in table I.
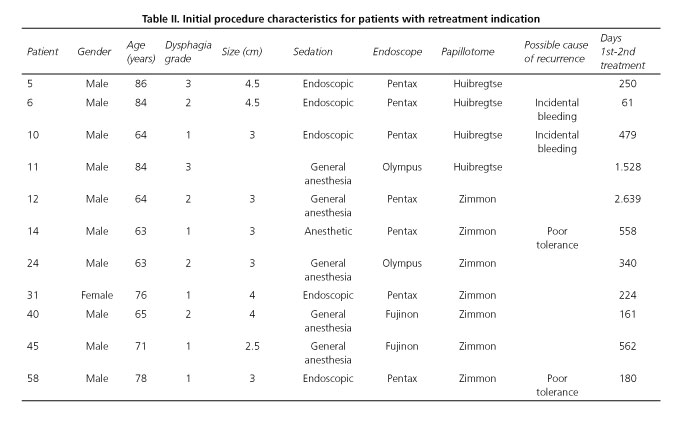
Eleven (19%) of these 57 patients had some sort of symptom recurrence and were retreated at 20 (2-88) months (Table II). A patient with no dysphagia suffering from repeat pneumonia was retreated with a second flexible CPMST at 8 months, but a bronchioloalveolar cell lung carcinoma was later diagnosed, which proved fatal 21 months after the initial CPMST procedure.
The remaining ten patients had dysphagia of varying grades (1-3). Four had received incomplete CPMST because of incidental bleeding or poor tolerability. However, among the variables analyzed only initial dysphagia grade was higher in the retreated (1.73 ± 0.79) versus the non-retreated (1.24 ± 0.60) group (p = 0.049), with no significant differences in gender, age, diverticular size, sedation type, endoscope type, or papillotome type for initial CPMST.
Seven symptom recurrence cases were subjected to a second flexible CPMST. This was complete for six. The remaining repeat CPMST was incomplete because of incidental bleeding (using a HP). In one patient with incomplete initial CPMST because of poor tolerability retreatment was aborted due to a problem with anesthesia and the patient rejected any further attempts of therapy. A patient with delayed recurrence (at 51 months) was retreated with hybrid septotomy under endoscopic control using a laparoscopic LigaSure® (Covidien, Mansfield, MA. USA) device. The remaining patient underwent surgical diverticulopexy.
We have long-term follow-up (31 [4-112] months) data available for 37 patients; dysphagia grades of 0.18 ± 0.45 were obtained, with the distribution shown in table I. Twenty-nine patients (78.4%) reported no dysphagia at all, and four had some difficulties with specific solid foods (usually beef) or solid drugs. Only one patient had overt solid and occasionally semisolid dysphagia; this patient rejected further therapy after a retreatment attempt was aborted because of an incident with anesthesia.
Two further deaths have occurred, one from cardiovascular disease and one from community-acquired pneumonia. All 5 confirmed demises involved patients older than 80 years at the time of CPMST, and 3 also had severe malnutrition. No follow-up data are available regarding the 3 patients older than 90 at CPMST.
Subgroup analysis
Sedation vs. anesthesia
Sedation was administered by the endoscopist/nurse for 25 (39%) subjects and by an anesthesiologist for the remaining, 30 of them (47%) using GA + OTI.
Tolerability was good for all but two patients who were sedated by an anesthesiologist and one sedated by the endoscopist/nurse. No anesthesia-related complications were recorded.
Three patients under GA + OTI (10%) required using a guidewire for DSC implantation, versus only one (3%) under sedation (p = NS).
Retreatment rate was 24% (6/25) for endoscopic sedation, 11% (1/9) for anesthetic sedation, and 17% (5/30) for GA + OTI (p = NS).
Huibregtse vs. Zimmon papillotome
In the initial 11 subjects CPMST was performed using a HP and in the subsequent 53 using a ZP.
Nine (82%) CPMSTs with HP had incidental bleeding and required APC. CPMST with ZP resulted in 5 (9.4%) incidental bleedings that were controlled with APC (p < 0.05).
Retreatment rate was 36% (4/11) in the HP group and 13% (7/53) in the ZP group (p = 0.064).
Pentax/Fujinon vs. Olympus gastroscope
In 50 cases Pentax gastroscopes were used, whereas Fujinon devices were used for 5 and Olympus scopes for 9 subjects.
In the OG group an esophageal perforation was recorded, this being the only serious complication directly related to CPMST.
Retreatment rate was 16.3% (9/55) in the PFG group and 22.2% (2/9) in the OG group (p = NS).
Overall, the 9 patients treated with an OG had 3 (33.3%) negative outcomes in total: 1 serious complication and 2 retreatment indications. Only 9 (16.3%) retreatment indications for clinical recurrence occurred amongst the 55 patients managed with a PFG.
Discussion
ZD is a low-prevalence condition, hence experience in its management is difficult to acquire. In 2002 we carried out the first Spanish FET, and the subsequent report of our three initial patients (11) made us a reference at the regional level, which has allowed our treating 64 patients over 13 years.
The first lesson learned was that therapy indication must be established according to patient general status, and particularly patient respiratory and nutritional status. In patients with severe malnutrition CPMST should be delayed until nutrition is improved by other means (e.g., gastrostomy) as any incidents or complications may become much more serious, even fatal, in the presence of malnutrition. Our female patient who had pharyngoesophageal perforation had a good general and nutritional status, and recovered from this serious complication. However, the two patients who had duodenal perforation and bronchoaspiration also had serious malnutrition and eventually died. Similarly, comorbidities potentially involving dysfunctional swallowing or chronic pneumonia (as in our patient with bronchioloalveolar cell carcinoma), which may compromise the clinical benefits of successful CPMST, should be assessed before the procedure is indicated (e.g., Parkinson's disease or stroke involving cranial nerves).
The potential use of sedation is a benefit of FET over rigid endoscopy. However, FET may also be performed under GA when indicated (nearly always because of lack of familiarity with the procedure by the anesthesiologist). In our series, OTI hinders but does not preclude flexible DSC implantation, with recourse to a guidewire being ocassionally needed. Acute diverticulitis, likely drug-related from intradiverticular retention of NSAIDs or bisphosphonates (12), represents a special challenge, our advice being that the procedure be delayed until acute inflammation subsides with conservative measures.
The gastroscope used must have a caliber ≤ 9.8 mm, as greater diameters hinder an alerady limited maneuverability within the DSC. Our preference for PFGs (with working channel on the lower portion of the visual field) over OGs (with working channel on the left side) is based on the fact that, ideally, this channel should emerge at the 6 o'clock position, which would provide an optimal axial alignment of the visual field and papillotome with the septum to be cut. The more this axis is offset, the worse visualization and more uncomfortable section become. Logically, this negative perception is greater for an endoscopist used to operate a PFG and not so significant for one used to operate an OG. Some objective evidence also supports preference for PFGs since OGs have a higher number of negative outcomes recorded. However, this must be made relative to the fact that all procedures were performed by an endoscopist not familiar with OGs.
Although most published series were reported before their being commercially available (5-8,13,14), a DSC renders the working field stable, and is therefore key to facilitate CPMST in a safe manner (15,16), much safer than with orogastric tubes and oblique-end hoods (17-19). Their disadvantage is that the diverticular flap hides the diverticular bottom, thus making CPMST length estimations challenging. This difficulty may be overcome in bigger ZDs by regularly introducing the endoscope between the septum and the flap. For smaller ZDs the papillotome's calibrated end may be used to estimate the length of the residual septum.
While other cutting methods were initially described, including APC (8,20) and hot forceps (6,19), most authors use Huibregtse needle-knife papillotomes (5,7,14,21). An HP is fitted with a very fine needle that is ideal for papillary precuts but, in our experience, inappropriate for CPMST. Because of the high current density transmitted cuts occur faster, are wider, and control is poorer, which in our series translated into 90% incidental bleedings requiring APC and prolonging CPMST. Bleeding incidence might have been lower with pure coagulation current (22), but we decided to change over to a ZP (with a thicker end allowing for better control), an option also considered by other authors (23). A ZP significantly reduces bleeding incidence in addition to providing better control with MPEC pulses with no need to swap it for an APC catheter. The potential attribution of a lower bleeding rate to an overcome learning curve with the ZP is unlikely since the endoscopist had considerable experience in using a HP for papillary precuts prior to his first CPMST procedure. However, that the learning curve may be responsible for the higher retreatment rate of subjects initially managed with a HP cannot be excluded.
Of late, several teams have reported their experience with the "hook-knife"(24), IT-knife 2 (25), O-HibrydKnife, (26) and scissor-shaped SB-knife (27,28), all of them designed to perform submucosal dissection. Hybrid CPMST approaches with cutting tools have also been used, associated with laparoscopic cutting and sealing using a harmonic scalpel (29) and the LigaSure® device (30). In the absence of comparison studies, we deemed the use of a ZP through a DSC as effective and safe as any other option, though cheaper.
Early complications included three mild events (chest pain and/or fever with normal x-rays) that were managed with conservative measures, and did not lengthen the predicted hospital stay. Only one serious complication was directly related to CPMST, namely a delayed pharyngoesophageal perforation event 24 hours after the procedure. A review of the procedure recordings showed that 5-mm safety limits had been surpassed, which made clip suturing mandatory (13). Causality cannot be attributed to the OG, but the device was at least partly responsible according to our subjective perception.
The 57 patients presenting for their first clinical and endoscopic review reported virtually complete clinical responses despite the presence of diverticular remnants in all but one of them (the patient undergoing complete CPMST to the advintial layer and then sutured with clips [Fig. 1C]). Eleven (19%) of these 57 patients developed relevant manifestations (mostly dysphagia) over a highly variable period of time (2 months-7 years). The one marker of higher retreatment probability was higher initial dysphagia grade. A second CPMST was scheduled for 9 cases but eventually performed in 8. The remaining two recurrences were managed with hybrid septotomy with LigaSure® and surgical diverticulopexy.
While cases amount to a mean of 5 per year, 18 patients were accumulated over the last 18 months, which has impacted on long-term follow-up. Among the 37 patients on long-term follow-up (with a mean of 2.5 years), 33 (89%) remain asymptomatic with minimal or no dietary restrictions. The only patient with overt manifestations has rejected further attempts at treatment.
To conclude, FET for ZD as performed by digestive endoscopists using conventional endoscopes and deep sedation or GA is a safe, effective procedure. Our present protocol, involving a flexible DSC, incision with a ZP, subtotal CPMST, and prophylactic suture with clips, provides excellent long-term clinical results with an acceptable retreatment rate and low serious morbidity.
Acknowledgments
I am grateful to doctors Pedro Escartín, Luis Abreu, José Luis Calleja, Yago González- Lama, Mariano González-Haba, Teresa Sala, Vicente Pons, Gontrand López-Nava, Susana Prados, Gabriela Pastor, Carlos Guarner, Josep Giné, Fernando García-Durán, Esperanza Tomás, Fernando Bermejo, José Luis Martínez-Albares, Alejandro Repiso, Manuel Romero, Jesús Espinel, and Juan Ramón Pineda for their entrusting the treatment of their patients to me, and for their help in the collection of data for this paper.
References
1. Belsey RH. Functional disease of the esophagus. J Thoracic Cardiovasc Surg 1966;52:164-88. [ Links ]
2. Ellis FH, Schlegel JF, Lynch VP, et al. Cricopharyngeal myotomy for pharyngo-esophageal diverticulum. Ann Surg 1969;170:340-9. DOI: 10.1097/00000658-196909010-00004. [ Links ]
3. Dohlman G, Mattson O. The endoscopic operation for hypopharingeal diverticula: A roentgen-cinematographic study. Arch Otolaryngol 1960;71:744-52. DOI: 10.1001/archotol.1960.03770050004002. [ Links ]
4. Collard IM, Otte IB, Kestens PJ. Endoscopic stapling technique of esophago-diverticulostomy for Zenker's diverticulum. Ann Thorac Surg 1993;56:573-6. DOI: 10.1016/0003-4975(93)90906-X. [ Links ]
5. Ishioka S, Sakai P, Maluf-Filho F, et al. Endoscopic incision of Zenker's diverticula. Endoscopy 1995;27:433-7. DOI: 10.1055/s-2007-1005736. [ Links ]
6. Mulder CJJ, Den Hartog G, Robijn RJ, et al. Flexible endoscopic treatment of Zenker's diverticulum: A new approach. Endoscopy 1995;27:438-42. DOI: 10.1055/s-2007-1005737. [ Links ]
7. Hashiba K, De Paula AL, Da Silva JGN, et al. Endoscopic treatment of Zenker's diverticulum. Gastrointest Endosc 1999;49:93-7. DOI: 10.1016/S0016-5107(99)70452-9. [ Links ]
8. Mulder CJJ. Zenker's diverticulum: Treatment with a flexible endoscope. Gastrointest Endosc 1999;50:596-7. DOI: 10.1016/S0016-5107(99)70097-0. [ Links ]
9. Dzeletovic I, Ekbom DC, Baron TH. Flexible endoscopic and surgical management of Zenker's diverticulum. Expert Rev Gastroenterol Hepatol 2012;6:449-66. DOI: 10.1586/egh.12.25. [ Links ]
10. Dakkak M, Bennett JR. A new dysphagia score with objective validation. J Clin Gastroenterol 1992;14:99-100. DOI: 10.1097/00004836-199203000-00004. [ Links ]
11. De la Morena E, Pérez-Arellano E, Carreño R, et al. Tratamiento endoscópico del divertículo de Zenker. Cir Esp 2005;78:256-9. DOI: 10.1016/S0009-739X(05)70928-3. [ Links ]
12. Sharma R, DeCroos AJ. Zenker's diverticulitis secondary to alendronate ingestion: A rare cause of recurrent dysphagia. Gastrointest Endosc 2011;73:368-70. DOI: 10.1016/j.gie.2010.10.043. [ Links ]
13. Tang SJ, Jazrawi SF, Chen E, et al. Flexible endoscopic clip-assisted Zenker's diverticulotomy: The first case series. Laryngoscope 2008;118:1199-205. DOI: 10.1097/MLG.0b013e31816e2eee. [ Links ]
14. Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: The Mayo Clinic experience. Mayo Clin Proc 2010;85:719-22. DOI: 10.4065/mcp.2009.0663. [ Links ]
15. Evrard S, Le Moine O, Hassid S, et al. Zenker's diverticulum: A new endoscopic treatment with a soft diverticuloscope. Gastrointest Endosc 2003;58:116-20. DOI: 10.1067/mge.2003.311. [ Links ]
16. Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker's diverticulotomy: Cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007;39:146-52. DOI: 10.1055/s-2007-966140. [ Links ]
17. Sakai P, Ishioka S, Maluf-Filho F, et al. Endoscopic treatment of Zenker's diverticulum with an oblique-end hood attached to the endoscope. Gastrointest Endosc 2001;54:760-3. DOI: 10.1067/mge. 2001.119606. [ Links ]
18. Costamagna G, Mutignani M, Tringali A, et al. Treatment of Zenker's diverticulum with the help of a plastic hood attached to the endoscope. Gastrointest Endosc 2002;56:611-2. DOI: 10.1016/S0016-5107(02)70470-7. [ Links ]
19. Christiaens P, De Roock W, Van Olmen A, et al. Treatment of Zenker's diverticulum through a flexible endoscope with a transparent oblique-end hood attached to the tip and monopolar forceps. Endoscopy 2007;39:137-40. DOI: 10.1055/s-2006-945118. [ Links ]
20. Rabenstein T, May A, Michel J, et al. Argon plasma coagulation for flexible endoscopic Zenker's diverticulotomy. Endoscopy 2007;39:141-5. DOI: 10.1055/s-2007-966164. [ Links ]
21. Vogelsang A, Preiss C, Neuhaus H, et al. Endotherapy of Zenker's diverticulum using the needle-knife technique: Long term follow-up. Endoscopy 2007;39:131-6. DOI: 10.1055/s-2006-944657. [ Links ]
22. Mulder CJJ, Costamagna G, Sakai P. Zenker's diverticulum: Treatment using a flexible endoscope. Endoscopy 2001;33:991-7. [ Links ]
23. Huberty V, El Bacha S, Blero D, et al. Endoscopic treatment for Zenker's diverticulum: Long-term results. Gastrointest Endosc 2013;77:701-7. DOI: 10.1016/j.gie.2012.12.008. [ Links ]
24. Repici A, Pagano N, Romeo F, et al. Endoscopic flexible treatment of Zenker's diverticulum: A modification of the needle-knife technique. Endoscopy 2010;42:532-5. DOI: 10.1055/s-0029-1244163. [ Links ]
25. Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker's diverticulum: A case series. Gastrointest Endosc 2014;79:168-70. DOI: 10.1016/j.gie.2013.07.012. [ Links ]
26. Curcio G, Granata A, Bertani A, et al. Insulated-tip dissecting knife to treat Zenker's diverticulum: Is insulation the key? Gastrointest Endosc 2014;80:537-8. [ Links ]
27. Ramchandani M, Reddy N. New endoscopic "scissors" to treat Zenker's diverticulum. Gastrointest Endosc 2013;78:645-8. DOI: 10.1016/j.gie.2013.06.003. [ Links ]
28. Iacopini F, Costamagna G, Scozzaro A. Scissor-shaped diatermic knife with diverticuloscope assistance for Zenker's diverticulum septotomy. Gastrointest Endosc 2014;79:540-2. DOI: 10.1016/j.gie.2013.10.022. [ Links ]
29. Hondo FY, Maluf-Filho F, Giordano-Nappi JH, et al. Endoscopic treatment of Zenker's diverticulum by harmonic scalpel. Gastrointest Endosc 2001;74:666-71. DOI: 10.1016/j.gie.2011.05.007. [ Links ]
30. Noguera J, Dolz C, Vilella A, et al. Transoral endoluminal approach to Zenker's diverticulum using Ligasure™. Early clinical experiencie. Rev Esp Enferm Dig 2014;106:137-41. [ Links ]
![]() Correspondence:
Correspondence:
Emilio J. de la Morena Madrigal.
Department of Digestive Diseases.
Hospital Sanitas La Zarzuela.
C/ Pléyades, 25.
28023 Madrid, Spain
e-mail: emorena@sanitas.es
Received: 11-10-2015
Accepted: 14-02-2016











 texto en
texto en