Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.6 Madrid jun. 2016
Clinical Practice Guideline: Irritable bowel syndrome with constipation and functional constipation in the adult
Guía de Práctica Clínica: Síndrome del intestino irritable con estreñimiento y estreñimiento funcional en adultos
Fermín Mearin1, Constanza Ciriza2, Miguel Mínguez3, Enrique Rey4, Juan José Mascort5, Enrique Peña6, Pedro Cañones7 and Javier Júdez8, on behalf of the Sociedad Española de Patología Digestiva (SEPD), Sociedad Española de Medicina de Familia y Comunitaria (semFYC), Sociedad Española de Médicos de Atención Primaria (SEMERGEN) and Sociedad Española de Médicos Generales y de Familia (SEMG)
1 Coordinator of the CPG. Roma IV Bowel Disorders Committee. Member of AEG. Centro Médico Teknon. Barcelona, Spain.
2 Functional Gastrointestinal Disorders Group. SEPD. Hospital Universitario 12 de Octubre. Madrid, Spain.
3 Member of AEG and SEPD. Hospital Clínico Universitario. Universitat de Valencia. Valencia, Spain.
4 SEPD. Hospital Clínico Universitario San Carlos. Madrid, Spain.
5 Scientific Department. semFYC.
6 Coordinator. Digestive Diseases. SEMERGEN.
7 Coordinator. Digestive Diseases. SEMG.
8 Knowledge Management Department. SEPD
ABSTRACT
In this Clinical Practice Guideline we discuss the diagnostic and therapeutic approach of adult patients with constipation and abdominal complaints at the confluence of the irritable bowel syndrome spectrum and functional constipation. Both conditions are included among the functional bowel disorders, and have a significant personal, healthcare, and social impact, affecting the quality of life of the patients who suffer from them. The first one is the irritable bowel syndrome subtype, where constipation represents the predominant complaint, in association with recurrent abdominal pain, bloating, and abdominal distension. Constipation is characterized by difficulties with or low frequency of bowel movements, often accompanied by straining during defecation or a feeling of incomplete evacuation. Most cases have no underlying medical cause, and are therefore considered as a functional bowel disorder. There are many clinical and pathophysiological similarities between both disorders, and both respond similarly to commonly used drugs, their primary difference being the presence or absence of pain, albeit not in an "all or nothing" manner. Severity depends not only upon bowel symptom intensity but also upon other biopsychosocial factors (association of gastrointestinal and extraintestinal symptoms, grade of involvement, and perception and behavior variants). Functional bowel disorders are diagnosed using the Rome criteria. This Clinical Practice Guideline has been made consistent with the Rome IV criteria, which were published late in May 2016, and discuss alarm criteria, diagnostic tests, and referral criteria between Primary Care and gastroenterology settings. Furthermore, all the available treatment options (exercise, fluid ingestion, diet with soluble fiber-rich foods, fiber supplementation, other dietary components, osmotic or stimulating laxatives, probiotics, antibiotics, spasmolytics, peppermint essence, prucalopride, linaclotide, lubiprostone, biofeedback, antidepressants, psychological therapy, acupuncture, enemas, sacral root neurostimulation, surgery) are discussed, and practical recommendations are made regarding each of them.
Key words: Irritable bowel syndrome. Constipation. Abdominal discomfort. Adults. Primary care. Digestive diseases. Clinical practice guideline.
RESUMEN
En esta Guía de Práctica Clínica analizamos el manejo diagnóstico y terapéutico de pacientes adultos con estreñimiento y molestias abdominales, bajo la confluencia del espectro del síndrome del intestino irritable y el estreñimiento funcional. Ambas patologías están encuadradas en los trastornos funcionales intestinales y tienen una importante repercusión personal, sanitaria y social, afectando a la calidad de vida de los pacientes que las padecen. La primera es el subtipo de síndrome del intestino irritable en el que el estreñimiento es la alteración deposicional predominante junto con dolor abdominal recurrente, hinchazón y distensión abdominal frecuente. El estreñimiento se caracteriza por la dificultad o la escasa frecuencia en relación con las deposiciones, a menudo acompañado por esfuerzo excesivo durante la defecación o sensación de evacuación incompleta. En la mayoría de los casos no tiene una causa orgánica subyacente, siendo considerado un trastorno funcional intestinal. Son muchas las similitudes clínicas y fisiopatológicas entre ambos trastornos, con respuesta similar del estreñimiento a fármacos comunes, siendo la diferencia fundamental la presencia o ausencia de dolor, pero no de un modo evaluable como "todo o nada". La gravedad de estos trastornos depende no sólo de la intensidad de los síntomas intestinales sino también de otros factores biopsicosociales: asociación de síntomas gastrointestinales y extraintestinales, grado de afectación, y formas de percepción y comportamiento. Mediante los criterios de Roma, se diagnostican los trastornos funcionales intestinales. Esta Guía de Práctica Clínica está adaptada a los criterios de Roma IV difundidos a finales de mayo de 2016 y analiza los criterios de alarma, las pruebas diagnósticas y los criterios de derivación entre Atención Primaria y Aparato Digestivo. Asimismo, se revisan todas las alternativas terapéuticas disponibles (ejercicio, ingesta de líquidos, dieta con alimentos ricos en fibra soluble, suplementos de fibra, otros componentes de la dieta, laxantes osmóticos o estimulantes, probióticos, antibióticos, espasmolíticos, esencia de menta, prucaloprida, linaclotida, lubiprostona, biofeedback, antidepresivos, tratamiento psicológico, acupuntura, enemas, neuroestimulación de raíces sacras o cirugía), efectuando recomendaciones prácticas para cada una de ellas.
Palabras clave: Síndrome del intestino irritable. Estreñimiento. Molestia abdominal. Adultos. Atención primaria. Enfermedades digestivas. Guía de práctica clínica.
Conceptual aspects, impact and pathophysiology
1. Why are irritable bowel syndrome with constipation and functional constipation in the adult jointly approached?
Irritable bowel syndrome (IBS) and functional constipation (FC) are two functional bowel disorders (FBDs) (1,2). Therefore, both conditions have in common that their cause is not explained by morphological, metabolic or neurological changes that may be shown by routine diagnostic techniques. IBS may be divided, according to the predominant bowel habit change, into IBS with constipation (IBS-C) and IBS with diarrhea (IBS-D); when both change types (constipation and diarrhea) alternate, the condition is referred to as mixed-type IBS (IBS-M), and when bowel habit lies somewhere between constipation and diarrhea the condition is denoted indeterminate IBS (IBS-I) (1,2).
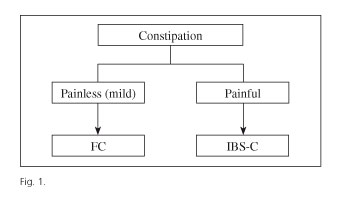
While IBS-C and FC are different FBDs from a conceptual perspective, they may in practice become very similar, even indistinguishable conditions (3-5). In both, constipation is the primary symptom, in association with abdominal bloating/distension. The presence of abdominal pain more than once a week and the temporal relationship of pain with defecation theoretically differentiate IBS-C from FC (1). However, patients with FC may feel some pain, and it is not always easy to determine temporal relationships (3). In fact, IBS-C and FC are part of a spectrum where patients with severe abdominal pain and constipation are on one end, and patients with constipation and no pain at all are on the other end; in practice, most cases fall somewhere in between. More rationally, this type of FBD could perhaps be classified as follows: painful constipation (similar to IBS-C) and unpainful constipation (similar to FC) (Fig. 1).
In addition to the above conceptual and clinical similarities, IBS-C and FC share several pathogenic mechanisms, and both have responded favorably to the same drugs (6-11).
All the above led us to develop a set of CPG to jointly approach IBS-C and FC. Doubtless, both conditions share more similarities than differences.
2. What is irritable bowel syndrome?
IBS is characterized by the presence of recurrent abdominal pain associated with bowel habit changes, whether in the form of constipation or diarrhea or combining both; bloating and abdominal distension are very common in IBS (1,2). According to the Rome IV criteria (2), IBS is diagnosed based on the presence of recurrent abdominal pain, which must be present for at least one day weekly, with two or more of the following characteristics: a) it is associated with defecation; b) it relates to a change in bowel movement frequency; and c) it is linked to a change in stool consistency. As per duration requirements, these criteria must have been met for the past three months, and symptoms must have had their onset at least six months prior to the diagnosis (1,2).
The overlapping of IBS with other FBDs (such as FC or functional diarrhea), other functional digestive extraintestinal disorders (such as functional dyspepsia or functional heartburn), or other non-digestive disorders (such as fibromyalgia or interstitial cystitis) is very common (12,13).
The diagnosis should be based on the characteristic symptoms systematized by the Rome IV criteria (Tables I and II, algorithm 1), but this is no excuse to do without the pertinent examinations to establish a differential diagnosis with some organic conditions that may have similar manifestations.
3. What is irritable bowel syndrome with constipation?
IBS-C is the IBS subtype where constipation is the predominant bowel habit. Stool characteristics allow to categorize IBS into subtypes using the Bristol Stool Scale (14) (Fig. 2). According to the proportion of each fecal type during the days when feces are abnormal IBS may be determined to be IBS-C, IBS-D or IBS-M. For IBS-C, over 25% of bowel movements should involve type-1 or type-2 stools, with fewer than 25% resulting in type-6 or type-7 stools (1,2) (Table I).
4. What is functional constipation?
Constipation is characterized by difficulty in passing stools or a low frequency of bowel movements, often accompanied by straining during defecation or a feeling of incomplete evacuation (1,2). In most cases no underlying physical cause is to be found, and the condition is considered as a FBD. According to the Rome IV criteria (Table II), FC is defined as the presence of two or more of the following during the previous 3 months: a) defecatory straining (≥ 25% bowel movements); b) hard or lumpy stools (≥ 25% bowel movements); c) a feeling of incomplete evacuation (≥ 25% bowel movements); d) defecatory obstruction (≥ 25% bowel movements); e) manual maneuvers to facilitate defecation (≥ 25% bowel movements); and f) fewer than 3 spontaneous complete bowel movements per week. Symptoms must be present for at least 6 months before the diagnosis, diarrhea must not be present except after using a laxative, and IBS criteria must not be met (1).
The American Gastroenterological Association (AGA) prefers a simpler though highly similar definition: "Unsatisfactory defecation characterized by infrequent stools, difficult stool passage or both for at least 3 months. Difficult stool passage includes straining, a sense of incomplete evacuation, hard/lumpy stools, prolonged time to pass stool, or need for manual maneuvers to pass stool" (15).
However, these definitions have been established through medical consensus and expert opinions, and the views of patients regarding their constipation are also important. Thus, in a population-based study in the USA, the complaints reported by a total of 557 subjects were as follows: 79% straining, 74% gas, 71% hard feces, 62% abdominal discomfort, 57% unfrequent stools, 57% abdominal distension, and 54% sensation of incomplete evacuation (16).
5. What similarities and differences are there between irritable bowel syndrome with constipation and functional constipation?
As discussed above, there are many clinical similarities between IBS-C and FC: they are more common in people with similar characteristics (middle-aged women), constipation is obviously present (also abdominal bloating/distension), and the latter's response to commonly-used drugs is also similar. Importantly, constipation is similarly characterized for both these FBDs (3). The key difference between both lies in the presence or absence of pain, but again this is an inconsistent finding that cannot be assessed on an all-or-nothing basis.
As regards the pathophysiology, constipation causes are also common to both conditions: colonic motility changes, difficulty in expelling stools, insufficient abdominal press function, and a combination of the above. None of the aforementioned causes, however, may be identified in a significant number of (particularly IBS-C) cases.
The crucial pathophysiological difference may well lie in different visceral sensitivities: colonic hypersensitivity is more common in IBS, and rectal hyposensitivity is more often seen in FC (17-19).
6. What is the clinical, social, and financial significance of irritable bowel syndrome with constipation and functional constipation?
Some physicians consider IBS-C and FC to be trivial conditions, but their personal, healthcare, and social impact is highly significant. Health-related quality of life (HRQoL) is considerably impaired in patients with IBS, as shown by relevant reviews (20,21). IBS-related costs, in turn, are also significant. Suffice it to say that 3.5 million people visit doctors for this problem each year in the USA, which represents a yearly cost of 20,000 million USD (22). Data obtained from Europe, specifically from Spain, also show an increase in both direct and indirect costs for patients with IBS-C (23).
Regarding the impact of FC on the daily lives of patients, 69% consider it impairs their academic or occupational performance (16), the condition being a relevant cause of absenteeism in severe cases (mean loss of 2.4 activity days/month), and of reduced productivity (16).
Other studies have confirmed the condition's social impact on comparing FC patient data to the general population (24). All this carries an enormous health care cost, both direct and indirect, for FC. In the USA, FC accounts for approximately 2.5 million visits and 92,000 hospitalizations yearly, with a cost of nearly 7,000 million USD in diagnostic assessments (24,25).
The findings of a systematic review in 2010 may well illustrate the HRQoL issue in FC. Ten studies were identified that used various generic health questionnaires: seven used the SF-36 (Short-Form 36), two the PGWBI (Psychological General Well Being Index), and one the SF-12 scales (26). Virtually all SF-36 domains were impaired in FC patients as compared to healthy controls; as expected, differences were greater for patients cared for in the outpatient versus the inpatient setting.
When the HRQoL of patients with constipation is compared to that of patients with other common conditions the results are amazing (26). The impact of FC on physical aspects is greater for patients requiring specialist care than for patients with ulcerative colitis (stable or otherwise), stable Crohn's disease, or other non-digestive conditions such as chronic allergy or back pain.
7. How can the severity of irritable bowel syndrome with constipation and of functional constipation be established?
Severity in FBDs, including IBS and FC, not only depends on intestinal bowel symptoms' intensity but also on other biopsychosocial factors (association of gastrointestinal and extraintestinal symptoms, grade of impairment, and perception and behavior variants). Thus, both visceral and central physiological factors play a role in IBS severity. Severity, in turn, directly impacts quality of life, and must be considered in the making of diagnostic and therapeutic decisions (27,28).
Severity in IBS, as well as in other FBDs, is usually established in two ways: a) using an individual symptom scale (e.g., mild, moderate, severe, extreme); or b) using a combination of various symptoms or attitudes (e.g., abdominal pain together with stool frequency and consistency, defecatory urgency, impact on quality of life, usage of health care resources, disability level).
The Irritable Bowel Syndrome Severity Scoring System (IBS-SSS) is the questionnaire most widely used for the assessment of IBS severity (29). It surveys the intensity of 5 different items along 10 days: abdominal pain, distension, stool frequency, stool consistency, and interference with daily activities. Each item is scored on 0-to-100 visual analog scale, and all 5 scores are then added up. The IBS-SSS tool has been translated into Spanish and validated (30).
Diagnosis
8. How many pathophysiological types of functional constipation (with or without irritable bowel syndrome) are there?
FC is classified according to the pathophysiological mechanism involved in three catergories (1,31-34).
1. Patients with functional defecatory disorders (Table III): impaired rectal emptying from inadequate rectal propulsion or abnormal relaxation in the striated muscle responsible for opening the anal canal (relaxation deficiency, paradoxical contraction or dyssynergic defecation) may be detected. Both dysfunctions may be associated and often result in diminished rectal sensitivity (hyposensitivity), structural pelvic floor defects (excessive perineal descent, rectocele, enterocele, intussusception, etc.) or in colonic motor disorders with delayed colonic transit time (CTT) (32).
2. Patients with slow colonic transit (SCT), where the time it takes the intestinal material to pass through the colon is longer than normal.
3. Patients with normal colonic transit (NCT). Diagnosing these pathophysiological subtypes requires functional diagnostic techniques that must be carried out in specialist centers.
Which functional studies allow to establish a diagnosis of defecatory dysfunction? Where and in which order should they be performed?
Three examination techniques help provide a diagnosis. While no consensus exists to unify their method, the presence of ineffective evacuation should be ascertained using at least two techniques (32).
Given its accessibility, simplicity, cost, absence of side effects, and diagnostic sensitivity and specificity, the balloon expulsion test must come always first (32,34-36). While no specialist facility is needed, its performance is challenging in a Primary Care or specialist outpatient setting. This test assesses a patient's ability to expel, under intimacy conditions, a water-filled balloon at body temperature and with enough volume to induce an urge to defecate. Expulsion within up to 1-2 minutes is deemed normal. In a non-controlled study of patients with FC this test was useful to identify defecatory dysfunction, with a sensitivity and specificity of 87.5% and 89%, respectively, and with a positive predictive value and a negative predictive value of 64% and 97%, respectively (37). Hence, the probability that a patient with normal test results may have a defecatory disorder is very low; however, a careful assessment of the anorectal function should be undertaken for any abnormal results. Most useful to this end is anorectal manometry (32,34-36), which records pressures along the rectum and anal canal both at rest and during spontaneous or induced defecation with an intrarectal balloon, assesses rectal responsiveness, and identifies anorectal reflex indemnity. In dyssynergic patients inadequate relaxation or paradoxical contraction at the anal canal is identified, as well as the presence or absence of enough intrarectal pressure to propel stools. Both the balloon expulsion test and anorectal manometry have, among others, the drawback of their performance with the anal canal permanently occupied by a tube, which is no guarantee that the defecatory maneuver will replicate what the individual experiences in his or her daily life. Therefore, if patient symptoms are not accounted for by the findings of these two tests, or any divergence is identified, a defecography study should be carried out (33,38). Besides function, this technique allows also the study of anorectal anatomy during the voluntary process of defecation. Two techniques are available: videofluoroscopy, which assesses and quantifies the ability to expel rectal contents (with the patient seated on a radio-translucent commode) and the presence of structural abnormalities in the sigma, rectum and anal canal, and magnetic resonance (MR) defecography, which also displays soft perirectal tissues and the genitourinary system in multiple anatomical planes, makes use of no ionizing radiation, and is less operator-dependent than videofluoroscopy. Both techniques must be performed in specialist units, and the interpretation of results should always be checked against patient symptoms before therapy decisions (particularly involving surgery) are made, given the high prevalence of morphological changes (rectocele, enterocele, intussusception) in otherwise normal individuals.
Which functional studies allow to establish a diagnosis of slow transit time constipation, and where should they be carried out?
Three techniques measure total and segmental CTT quantitatively: radiographic study with radiopaque markers (39), colonic scintigraphy after a meal (40) or the ingestion of an indium-marked capsule (111In-DTPA) (41), and the use of a wireless motility capsule (SmartPill®) (42). All these techniques should be carried out and interpreted in specialist units, with the study with radiopaque markers being most commonly ordered because of its wider accessibility. In Spain, a study in a high number of normal subjects provides reference values for radiological tests (39). The SmartPill® system, although costly, has shown a good correlation with radiological studies for the classification of patients with SCT versus NCT, entails no ionizing radiation, and also assesses motor activity throughout the gastrointestinal tract. This is very important when deciding to perform colon resection surgery for a patient with SCT, since motor disorders must be ruled out for the remaining intestine.
9. What is the clinical utility of knowing which is the pathophysiological type of functional constipation?
Early diagnosis of defecatory dysfunction resulting from dyssynergic pelvic floor is very useful in clinical practice because of the condition's prevalence and response to biofeedback (BFB) therapy as opposed to standard therapy (43-46), and because BFB returns slow CTT to normal in a high proportion of patients (43).
In patients without defecatory dysfunction, CTT will allow a less aggressive approach to therapy. Patients with NCT should never be treated with extreme measures, even less so with surgery. Furthermore, patients with SCT and no defecatory dysfunction commonly experience clinical worsening in response to fiber, and usually respond poorly to routine laxatives (including stimulant laxatives). In this subgroup of patients sacral nerve root neuromodulation (47), as well as subtotal colectomy with ileorectal anastomosis for highly selected cases, may effectively achieve satisfactory results (48,49).
10. May a patient experience changing symptoms and meet the criteria for both diagnoses (constipation-predominant IBS and functional constipation) at different times in his or her lifetime?
When Rome criteria are used, both diagnostic overlapping (specially with Rome criteria previous to Rome IV) and diagnostic changes within the same individual are very common over time. A highly relevant prospective study in Primary Care (PC) (n: 432 subjects; FC: 231, IBS-C: 201) showed that 89.5% of patients meeting IBS-C criteria also met FC criteria (as defined at the time in 2005), and 43.8% of patients with FC fully met IBS-C criteria; in up to one third of patients a change in diagnosis (FC to IBS-C and IBS-C to FC) was seen during a 12-month follow-up period (50).
11. What diagnostic tests are needed for the diagnosis of constipation-predominant irritable bowel syndrome and of functional constipation?
As discussed above, the diagnoses of IBS-C and FC are made using data from the medical records, which must meet the criteria established by expert group consensus (Rome) (2) (Tables I and II), or the AGA in the case of FC (31).
Once the specific criteria for the diagnosis of either condition (IBS-C or FC) have been confirmed, and given the key requirement that symptoms must not have an organic, metabolic or drug-related origin, the diagnostic tests needed to account for symptom functionality should be clearly laid out. Symptom-driven history taking and careful physical examination are mandatory, and should help exclude both intestinal and extraintestinal diseases (Table IV), or the use of symptom-inducing drugs (Table V). They will also reveal whether any alarm symptoms are present (Table VI) that could prompt the ordering of specific diagnostic tests.
In the absence of alarm criteria, which laboratory or imaging tests are key to rule out a metabolic or organic cause for patients meeting the consensus clinical criteria of IBS-C or FC?
Except for a cell blood count (CBC) to assess the presence of anemia and/or infection, tests such as electrolyte, thyroid hormone, and calcium levels, and complete blood chemistry (fasting glucose, urea, creatinine, etc.) neither have proven to have diagnostic usefulness, nor are they cost-effective (31,33,50). Thus, such tests should only be ordered when the parameters they measure are suspected to be abnormal.
The usefulness of plain abdominal X-rays (51,52) or opaque enema (53) to unveil discriminating morphological characteristics with respect to the normal population remains also to be demonstrated, and no evidence supports the usefulness of colonoscopy in patients with clinical constipation (54,55). As a result, consensus guidelines (31,33,38) do not recommend that laboratory or morphological studies be performed, unless risk criteria are met or an organic or metabolic condition is suspected.
Which studies should be ordered when alarm criteria are met?
In addition to specific testing according to the index alarm finding, colonoscopy should be used in most cases.
What sort of laboratory and imaging follow-up should be implemented for patients diagnosed with IBS-C or FC who have remained clinically stable, with no alarm signs or symptoms, for several years?
None. The only exception would be a patient meeting age-related colorectal cancer (CRC) screening criteria or the development of CRC in a family member. In such cases the relevant studies, that is, fecal occult blood (FOB) or colonoscopy, should be performed (33).
What sort of follow-up should a patient with a well-established diagnosis of IBS-C or FC who develops changes in symptom severity, frequency, or response to treatment undergo?
In absence of a plausible explanation for such changes, a search for the presence of some causal condition should be initiated on an individual basis. Following a new physical examination, the time when previous laboratory and morphological tests (if any) were performed should be considered, and changes in the family epidemiological characteristics should be recorded. Typically, both conditions include stages where symptom severity changes and patients perceive they may have an organic disease that remains insufficiently explored. Furthermore, the fact that FC and IBS-C are interchangeable diagnoses over time within one same individual when using Rome criteria should be taken into account; up to one third of patients with FC will meet the IBS-C criteria within on year, and vice versa (4), which should not prompt further diagnostic testing. Only alarm symptoms or signs should warrant additional tests.
Once a patient is diagnosed with IBS-C or FC, which functional studies should be carried out, and when? Are symptoms useful to suspect the pathogenic mechanism underlying FC?
Patients with constipation, with or without IBS criteria, may develop functional anorectal changes or colonic motor disorders that will not respond to routine measures, which makes specific functional testing mandatory (Algorithms 2 and 3). The most common functional anorectal disorder is dyssynergic defecatory dysfunction, which affects from 14.9% to 52.9% of patients with FC (36), and basically results from impaired anal opening at defecation or insufficient rectal propulsion during the expulsive phase. Early diagnosis is very important for this disorder, which requires a specific therapy (anorectal BFB). Data may be found in the medical records that, although nonspecific for the diagnosis of anorectal dyssynergia (56,57), are more common in this condition according to some studies anal pain at defecation (7), manual maneuvers to help in stool expulsion, defecatory straining, and anal blockage (58). Furthermore, there is a sign strongly associated with this disorder when elicited by experienced clinicians, namely the presence of a paradoxical anal contraction when the patient performs a defecatory maneuver during an anorectal digital exam (59,60). In the presence of this sign, as elicited by experts under intimacy conditions, a balloon expulsion test should be ordered; if abnormal, an anorectal manometry procedure should follow to confirm dyssynergia. However, since these requirements (expertise in dynamic digital exams, properly conditioned exploration rooms) are rarely found in daily clinical practice, guidelines suggest that these tests be ordered for any patient unresponsive to management with hygienic-dietary measures, lifestyle changes, and routine laxatives when dyssynergia is suspected from symptoms or an anal digital exam (33,36); more stringently, this should even be a mandatory requirement before ordering such tests for patients also refractory to serotonin agonists and secretagogues (38). For patients with conflictive balloon expulsion test and anorectal manometry findings, a fluoroscopic or MR defecography procedure should be ordered to assess the presence of occult structural changes (enterocele, intussusception, rectocele) and/or to confirm pelvic floor dysfunction during defecation maneuvers.
Some symptoms are more prevalent in patients with SCT FC according to some observational series: defecatory infrequency (58,61), constipation since childhood, and dependence on laxatives (61), but only stool consistency (very hard, Bristol < 3) has been shown to have predictive value for the diagnosis of SCT (sensitivity 85%, specificity 82%) (62). Presently, CTT must be studied in patients not responding to any therapy, preferrably once anorectal dyssynergy has been ruled out using the balloon expulsion test and anorectal manometry (31,33,38).
12. May functional constipation pathophysiological subtypes be diagnosed in the Primary Care setting? How?
According to diagnostic criteria, pathophysiological constipation subtypes require diagnostic techniques not available in the PC setting; however, some basic symptoms or signs have shown fairly good correlation with the results obtained using said techniques. Given the potential significance the prioritization of specific tests may have from a prognostic and therapeutic perspective, awareness and recognition of these symptoms and signs is crucial.
For a diagnostic approach to FC subtypes in PC, careful history taking and physical examination are key. During anamnesis the following should be elicited: defecatory pattern (stool frequency and consistency), associated symptoms and signs (pain, discomfort, distension, defecatory straining, feeling of incomplete evacuation, manual removal of stool, etc.), prior therapy history (lifestyle changes, dietary measures, laxatives, painkillers, antidepressants, etc.), and prior response to treatment. Physical examination must include a complete abdominal exploration, anal and perineal inspection, and dynamic rectal exam (with defecatory maneuver) (63).
As discussed above, symptoms may be elicited that, based on their differential prevalence, suggest dyssynergic defecation or SCT, and most importantly, the afore-mentioned sign, anal dyssynergy during anorectal digital examination, with predictive value (under expert hands) for the diagnosis of dyssynergic defecation.
Treatment
13. The importance of therapy compliance and general practical considerations regarding treatment options
As with any health condition, stringent compliance is essential for the effectiveness of prescribed therapy (Algorithms 4 and 5). In the present case, this includes adherence not only to drugs but also to hygienic-dietary measures (Table VII) and lifestyle changes, when appropriate.
Indeed, prescribing a therapy regime is not enough, as it will be useless unless the patient understands it, accepts it, and agrees to follow it. Therefore, this is not about patients obeying and complying with a prescription, but a trust-based patient-doctor relationship should be set up to promote cooperation, and the patient's active role in decision-making and responsibility regarding self care.
Therefore, in addition to objetively considering the best therapeutic options available, other aspects must be assessed to facilitate patient engagement and compliance. These include the following:
- Use drugs with simple dosage schemes; least possible number of doses or galenic formulations allowing simpler administration.
- Provide patient with simple, easy-to-understand written information and reminders.
- Provide compliance "diaries" to track adherence to medication or prescribed activities.
- Provide patient with information on the condition's pathophysiology according to his or her idiosyncracy and education level to promote involvement in self-care.
- Include family members and caregivers in all these strategies so that they may positively reinforce patient's behavior.
- Regularity is very important for constipation management. While some patients permanently medicate themselves with stimulant laxatives, others only use them intermittently and on an ad hoc basis for exacerbations; other patients avoid all treatments mistakenly believing that laxatives induce dependence or may be ultimately dangerous.
Finally, and importantly, nurses may play a highly effective role in health care education and in monitoring patient outcomes.
At any rate, attempts to assess a therapeutic regime's effectiveness will be useless if optimal patient adherence is not secured. Ineffectiveness will not improve with isolated or overall prescription changes unless patient's engagement can be gained.
14. Usefulness of aerobic exercise to improve: a) constipation; b) abdominal pain; and c) distension
Exercise is often empirically recommended to improve constipation and abdominal distension. Aerobic exercise is useful to maintain adequate bowel function and cut down stress (64).
Efficacy
A regular aerobic exercise schedule (walking, cycling) may be effective against constipation, and improvements in total and recto-sigmoid CTT have been reported (65). Benefits on abdominal distension and gas retention have also been observed (66), but to a lesser extent when compared to healthy subjects (67). Two additional studies reported overall symptom and fecal consistency improvement in IBS (68,69). Other aspects that may play a role in IBS, such as anxiety and depression, were also improved (68).
Limitations
Regular and moderate aerobic exercise according to patient fitness has no significant clinical limitations except in patients with impaired mobility. However, optimal intensity and duration remain to be established.
Practical recommendations
Regular aerobic exercise may help relieve constipation, favors intestinal gas evacuation, and improves distension; exercise recommendation to patients with IBS-C or FC seems thus advisable.
15. Does increased fluid ingestion improve constipation?
Most clinical guidelines recommend lifestyle changes including adequate fluid ingestion and a fiber-rich diet. Specifically, an ingestion of 1.5-2 l of liquids daily is recommended by some guidelines (49,70,71) and by more specific reviews about FC in the elderly (72).
Efficacy and limitations
In one randomized study, the intake of 2 l of water daily by patients with FC already on a fiber-rich diet improved defecatory frequency and reduced laxative needs (73). However, no clinical trials are available to demonstrate that fluid ingestion alone, without any concomitant measures, does improve constipation except for dehydrated patients (49,50).
Practical recommendations
While evidence is insufficient to recommend increased fluid intake, this approach is indeed somewhat beneficial for mild constipation when associated with adequate fiber ingestion in the diet.
16. Usefulness of fiber in the diet to improve: a) constipation; b) abdominal pain; and c) distension
Most guidelines recommend eating a fiber-rich diet to relieve constipation. A gradual increase in fiber is usually advised to prevent abdominal distension; the recommended amount of fiber is at least 25-30 g daily. However, while this measure may improve defecatory frequency and stool consistency, it also may worsen symptoms such as abdominal pain and distension.
Efficacy
A meta-analysis concluded that eating prunes (100 g/day) is beneficial for constipation, the effect reported being superior to that of Psyllium (74).
Another meta-analysis found that fiber-rich diet is useful to improve constipation but not so to relieve abdominal pain and distension in patients with IBS (75). In this same systematic review food-related soluble fiber is what benefitted patients with IBS.
Limitations
Although soluble fiber-rich foods may have some benefits for constipation, the same does not ring true for nonsoluble fiber. A meta-analysis of 5 studies with a total of 221 patients concluded that no differences exist in symptom relief between subjects taking insoluble fiber and subjects on a low-fiber diet or on placebo (relative risk [RR]: 1.02; 95% confidence interval [CI]: 0.82-1.27) (76). Furthermore, in patients with severe constipation and significantly slowed down CTT, high-fiber diet is ineffective and may worsen pain and distension (49).
Practical recommendations
Dieting on foods with high soluble fiber contents (such as prunes) has proven beneficial against mild constipation but not against abdominal pain and distension; these symptoms may in fact worsen in patients with IBS.
17. Usefulness of diets in constipation-predominant IBS and functional constipation to improve: a) constipation; b) abdominal pain; and c) distension
Approximately two thirds of patients with IBS believe their symptoms are triggered by some specific food.
Wheat sensitivity in the absence of celiac disease has been reported in some patients with IBS. A clinical trial that studied 920 patients with IBS symptoms found that one third of subjects worsened (increased abdominal pain and distension) after receiving wheat but not placebo (77). However, the role of non-celiac gluten sensitivity remains to be established: in a study in patients diagnosed with this condition and who met IBS criteria, randomized, blinded gluten administration at different concentrations could not be told apart from placebo (78).
While lactose malabsorption plays no role in constipation, it has been associated with abdominal pain and distension in patients with IBS. A systematic review analyzed the findings of 7 studies in IBS patients undergoing a lactose intolerance hydrogen breath test; over one third had lactose malabsorption, and lactose intolerance was more common among patients versus control subjects (79). However, patients with IBS often display gastrointestinal symptoms after eating dairy products even if lactose malabsorption cannot be demonstrated.
Furthermore, by extrapolating the intolerance hypothesis to several carbohydrates in IBS, diets free from oligosaccharides, disaccharides, monosaccharides, and fermentable polyols (FODMAP) have been proposed. In a randomized study, 41 patients with IBS received low FODMAP diet or their regular diet; 68% of patients on the low FODMAP diet reported adequate symptom control versus 23% of those on their regular diet (p = 0.005). Stool consistency did not change in either group (80). However, in a recent study low FODMAP diet was not superior to traditional dietary counseling for IBS (81).
Limitations
For side effects these diets may potentially induce malnutrition when sustained.
Practical recommendations
The role of some dietary components as symptom triggers or in the pathogenesis of IBS is of increasing interest. Gluten-free diet and low FODMAP diet seem to improve abdominal pain and distension but not constipation in IBS. Low FODMAP diet has not been assessed in patients with FC but its usefulness is unlikely. In short, the current evidence to support their routine use for IBS and FC in clinical practice is limited.
18. Usefulness of fiber supplementation to improve: a) constipation; b) abdominal pain; and c) distension. Which type of fiber? How is tolerability?
Mechanism of action
Dietary fiber supplements include several complex, poorly digestible carbohydrates that reach the colon unchanged to contribute to fecal bulk; they are partly fermented by the microbiota, which results in short-chain fatty acids, water and gas (hydrogen, methane, carbon dioxide). They are usually classified as soluble and insoluble fiber, according to their behavior in water solutions. The biological effects of fiber include CTT acceleration, increased biomass with colonic pH and microbiota changes, and potential changes in permeability and inflammation (82).
Efficacy
Several meta-analyses have reviewed the evidence on fiber for FC and IBS. All of them agree that drawing joint conclusions is challenging because of the studies' heterogeneous designs, varying goals, and poor quality. Overall, fiber is beneficial for constipation symptoms (stool numbers, defecatory straining) or secondary endpoints (use of laxatives); it is superior to placebo, especially soluble fiber (Psyllium), in the studies with both FC and IBS patients. The effects on other symptoms such as pain and distension remain unclear (83-85).
Adverse effects
The main adverse effects of dietary fiber derive from its potential to induce distension, usually as a result of gas from bacterial fermentation. Most clinical trials do not report this as relevant, but clinical practice shows this effect may be significant, particularly for patients with constipation associated with abdominal pain and distension (86).
Limitations
The use of dietary fiber supplements has no signifcant clinical limitations; they should be used with caution and clinical monitoring in bedridden patients or in individuals with severely disordered intestinal and colonic motility because of increased impaction risk.
Practical recommendations
The use of dietary or supplemental fiber is a rational first-line therapy for any patient with constipation, whether associated with abdominal discomfort or otherwise. Evidence is stronger regarding soluble fiber, and a therapy trial of 6 weeks usually suffices to assess efficacy (87). Attention must be paid not only to efficacy but also to tolerability, hence gradual increases in fiber amount are to be advised.
19. Usefulness of osmotic laxatives to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Osmotic laxatives contain non-absorbable ions or molecules that retain water in the bowel lumen. Polyethylene glycol (PEG), lactulose, and magnesium salts are most commonly used.
Efficacy
These laxatives improve constipation and fecal consistency, but abdominal pain and distension respond poorly. According to available studies, PEG is most supported by evidence, but magnesium salts are commonly used in clinical practice with satisfactory results.
Only two clinical trials have studied the use of osmotic laxatives (PEG) for IBS-C. The first one found no superiority over placebo (88). The second trial found benefits in terms of defecatory frequency but not of abdominal pain and distension (89).
As regards specific FC studies, 5 trials evaluated PEG versus placebo. PEG superiority was demonstrated with a NNT (number needed to treat) value of 3 (95% CI: 2-4). Lactulose was also superior to placebo with a NNT of 4 (95% CI: 2-7) (90).
In another systematic review comparing PEG and lactulose, PEG was superior in terms of number of weekly stools, fecal consistency, abdominal pain relief, and need for other drugs (91).
Adverse effects
Most common side effects include abdominal pain and distension, diarrhea, nausea, and vomiting. Hypersensitivity reactions (rash, urticaria or edema) have been reported in a few patients only. They have a good safety profile and may be used by the elderly, during pregnancy, and during breastfeeding. They can also be used in patients with liver or kidney failure. PEG has fewer side effects as compared to lactulose, and may be safely dosed for prolonged periods of time (up to 6 months). Regarding magnesium salts, the most commonly reported adverse effect is electrolyte imbalance; hence they must be used cautiously in patients with impaired renal function at risk for hypermagnesemia (71).
Limitations
The primary limitation of this type of laxatives is their poor relief of abdominal pain and distension, symptoms that are significant particularly for patients with IBS. PEG seems to be somewhat superior to lactulose in this respect, hence the latter is not advisable for patients with IBS-C (92).
Practical recommendations
Osmotic laxatives are useful to treat constipation but not so to manage abdominal pain and distension; therefore, they are first-line agents for FC but their usefulness is more limited in IBS-C. PEG is more effective than lactulose for symptom control, and also has fewer side effects, hence it is to be considered to be first-choice. These laxatives have a favorable safety profile and may be used in specific situations such as in the elderly, during pregnancy, and in patients with impaired liver and/or kidney function.
20. Usefulness of stimulant laxatives to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
These drugs promote water and electrolyte secretion in the colon or induce colonic persitalsis. They include diphenylmethanes (phenolphthalein, bisacodyl, sodium picosulfate) and anthraquinones (Senna, bearberry, Aloe vera).
Efficacy
Two clinical trials studied the efficacy of bisacodyl and sodium picosulfate. The first one included 247 patients who received 10 mg of bisacodyl versus 121 who received placebo once daily for 4 weeks. Bisacodyl was superior to placebo to improve constipation and its related symptoms, as well as quality of life (93). The second trial assessed the effectiveness of sodium picosulfate versus placebo; 131 patients received 10 mg of sodium picosulfate and 71 received placebo for 4 weeks. As above, sodium picosulfate was superior to placebo to improve constipation (94). Considering both studies combined, 42.1% of patients in the laxative group failed to respond to therapy, versus 78% of those receiving placebo; NNT was 3 (95% CI: 2-3.5) (90).
Adverse effects
Most common adverse effects include abdominal pain, diarrhea, nausea and vomiting. Allergic reactions have been described less often. Prolonged regimens may induce loss of fluids and electrolytes, hence they must be used cautiously in the elderly, in patients with heart failure, and in patients on diuretics or corticosteroids. Insufficient studies are available to support safety during pregnancy, therefore they cannot be recommended to pregnant women.
Limitations
As with osmotic laxatives, these drugs have not proven effective for abdominal pain and distension relief, and may even worsen these symptoms. Furthermore, they fail to induce a "predictable" bowel rhythm in many patients.
Practical recommendations
Stimulant laxatives are useful in the treatment of constipation, but no so good for abdominal pain and distension; therefore, their utility is limited in IBS-C. Their safety profile falls below that of osmotic laxatives.
21. Usefulness of probiotics to improve: a) constipation; b) abdominal pain; and c) distension
Mechanism of action
Probiotics are live bacteria that possess characteristics such as survival in the gastrointestinal tract, adherence to the intestinal epithelium, and intestinal flora modulation. They inhibit potentially pathogenic bacteria, and have various immunomodulating and immunostimulating effects; they promote immune cell proliferation, enhance phagocyte activity, and increase IgA production. All this determines their potential benefits in preventing infection, particularly by intestinal pathogens, as well as bacterial translocation (95-97).
Efficacy
According to the relevant systematic reviews and meta-analyses, the usefulness of probiotics to relieve overall symptoms (defecation, bloating, pain improvement) in patients with IBS remains unclear, with studies that find positive results and studies that find no significant differences.
Regarding FC, evidence is even poorer, given the heterogeneity and biases found in the relevant studies (90,98-100).
Adverse effects
No study has ever found side effects with the use of probiotics in these patients.
Limitations
No limitations to the use of probiotics are found in any study.
Practical recommendations
Given the current lack of evidence to support their use because of conflicting results regarding their effectiveness to relieve abdominal pain and distension, and to improve bowel movements in patients with IBS-C and FC, as of today we cannot recommend their use in these patients.
22. Usefulness of antibiotics to improve: a) constipation; b) abdominal pain; and c) distension
Mechanism of action
Rifaximin is a synthetic antibiotic derived from rifamycin and active against Gram-positive and Gram-negative germs, as well as both aerobic and anaerobic bacteria; it is not absorbed by the intestinal mucosa (< 0.01% following an oral dose), hence it has intraluminal activity and no systemic effects. It prevents pathogens from adhering to the bowel mucosa and from invading epithelial cells by binding microbial RNA-polymerase subunit β, thus inhibiting transcription and the synthesis of ribonucleis acid (RNA) (101).
Efficacy
Rifaximin seems to reduce distension, flatulence and abdominal pain in patients with IBS without constipation, according to the relevant studies (90,101-103). One study suggests its potential role for patients with IBS-C (104).
No studies have assessed rifamixin effects on FC.
Limitations. Adverse effects and contraindications
Studies and reviews do not report major side effects or adverse events seen more frequently than with placebo (3-5).
Practical recommendations
Evidence is currently insufficient to support recommendations regarding the use of rifaximin in patients with FC or IBS-C; however, the drug may reduce bloating and flatulence.
23. Usefulness of spasmolytics to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Spasmolytics have been traditionally used for the empiric management of IBS based on the fact that colonic smooth muscle contraction contributes to IBS manifestations, particularly pain. They include 3 major types: calcium channel blockers (otilonium and pinaverium), direct smooth muscle relaxants (mebeverine), and antimuscarinic/anticholinergic agents (hyoscine, cimetropium, dicyclomine hydrochloride).
Efficacy
Spasmolytics are superior to placebo for improving IBS symptoms, especially abdominal pain and distension (38% in the placebo group and 56% in the antispasmodic group; OR: 2.13 [95% CI: 1.77-2.58]) (105). The effect of individual spasmolytics is variable and difficult to interpret, as only a reduced number of studies have assessed each of the 12 drugs available. Among them, otilonium bromide (5 trials) and hyoscine (3 trials) had evidence of efficacy (76). Cimetropium bromide, pinaverium bromide, and dicyclomine hydrochloride have also demonstrated benefits to some extent (90). However, study heterogeneity is to be considered. Other assessed spasmolytics did not better than placebo.
Another multicenter clinical trial also revealed that patients receiving otilonium bromide were less likely to present with symptom recurrence versus placebo (106).
Adverse effects
Fourteen per cent of patients treated with antispasmodics reported side effects versus 9% of those receiving placebo. Most commonly reported side effects included dry mouth, dizziness, and blurred vision, with no serious adverse event reported. The relative risk of having an adverse effect versus placebo was 1.61 (95% CI: 1.08-2.39) (76,90).
Spasmolytics with greater anticholinergic activity may induce visual changes, urine retention, constipation, and dry mouth. They must be dosed with caution in elderly patients with a history of acute myocardial infarction and hypertension. Their use during pregnancy and breastfeeding is not recommended, as their safety remains to be established in such situations.
Limitations
Spasmolytics are useful to relieve pain and distension, but have no effect on constipation.
Practical recommendations
Spasmolytics are effective against abdominal pain and distension in patients with IBS, with a favorable safety profile. Side effects are uncommon. However, those with greater anticholinergic effects may induce adverse events at high doses.
24. Usefulness of peppermint essence to improve: a) constipation; b) abdominal pain; and c) distension
Mechanism of action
Peppermint essence, also commonly denominated peppermint oil, has spasmolytic properties and may modulate pain by attenuating visceral hypersensitivity.
Efficacy
Two systematic reviews show an effect superior to placebo's in the management of pain in patients with IBS (76,90). The most recent review included 5 trials for a total of 482 patients (107-111); it showed a statistically significant positive effect of peppermint oil versus placebo, with a NNT of 3 (95% CI: 2-4) (90). However, there was significant heterogeneity among studies.
Adverse effects
The above-mentioned studies reported no significant side effects versus placebo. Peppermint essence usually has no adverse effects at standard doses, but allergic reactions, heartburn, and headache have been described. The safety profile during pregnancy and breastfeeding is unknown at the standard doses given for IBS, so it cannot be recommended in such situations.
Limitations
As with other antispasmodic agents, this compound has not demonstrated effect on constipation.
Practical recommendations
Peppermint essence has proven effective for the management of pain and distension in patients with IBS with few adverse effects.
25. Usefulness of prucalopride to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Serotonin (5-HT) plays a key role in the gastrointestinal tract, where it affects the secretory, motor, and sensorial functions. Seven 5-HT receptor subtypes may be found in the gut. Of these, receptor 5-HT4 favors intestinal secretion, and enhances peristalsis and bowel transit (112). Prucalopride is a highly-selective 5-HT4 agonist that stimulates intestinal motility (113).
Efficacy
In phase-3 trials prucalopride was superior to placebo for improving constipation, abdominal pain and abdominal distension, as well as quality of life (114-116).
In a systematic review of 8 clinical trials, the response to prucalopride for constipation improvement was superior to the response elicited with placebo (therapy failed in 71% of patients with prucalopride and in 87.4% with placebo), but significant heterogeneity was identified amongst studies (90).
A meta-analysis including 9 trials also showed prucalopride to be effective for constipation with at least 3 bowel movements weekly (RR: 1.63; 95% CI: 1.07-2.49). It also improved quality of life (RR: 1.51; 95% CI: 1.07-2.11) and stool consistency (mean difference versus the control group 9.16; 95% CI: 7.28-11.03) (117). It seems to be as well a useful drug against refractory constipation in the elderly, according to the results obtained in a phase-2 clinical trial (118).
Furthermore, its potential role in other motility disorders that manifest with constipation, including chronic intestinal pseudo-obstruction, must be highlighted, even though evidence is now scarce. Prucalopride improved abdominal pain and distension in patients with this condition (119).
Further studies are needed to assess the combination of prucalopride with other drugs, such as linaclotide or lubiprostone, in patients with severe constipation.
Adverse effects
The drug is safe and well tolerated. Most common side effects include headache, nausea, abdominal pain, and diarrhea. It has an excellent cardiac safety profile because of its selective affinity for intestinal 5-HT4 receptors. However, it must be used with caution in patients with advanced renal failure or seriously impaired liver function. Prucalopride is not recommended during pregnancy (category C drug) or breastfeeding.
Limitations
The drug is not commercially available in Spain with the indication of IBS-C. However, from all the above, it may play a role in patients with severe IBS-C failing to respond to other therapies.
Practical recommendations
Prucalopride is effective in the treatment of FC unresponsive to other drugs; to a lesser extent, it also improves pain and distension in these patients with a good safety profile. It may be used in the elderly with refractory constipation, where the use of half doses (1 mg) is advisable.
26. Usefulness of linaclotide to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Linaclotide is a guanylate cyclase C agonist that increases intracellular cyclic guanosine monophosphate (cGMP) levels in the enterocyte. Intracellularly, cGMP increases bicarbonate and chloride secretion unto the bowel lumen, and diffuses to the extracellular compartment to inhibit sensory nerve terminal activity. From a pharmacodynamic standpoint, its ultimate effect is increased intraluminal secretion leading to enhanced transit and a visceral analgesic action, with reduced sensory thresholds to mechanical distension (120,121).
Efficacy
Based on the clinical trials and meta-analyses that compared linaclotide to placebo (122,123) both in patients with IBS-C (RR: 1.95; 95% CI: 1.3-2.9, based on 7 studies) and in patients with FC (RR: 4.26; 95% CI: 2.80-6.47, based on 3 studies), linaclotide is clearly effective for relieving constipation symptoms with a NNT of 7 (95% CI: 5-11) (122) in both groups, with highly homogeneous results across studies. Linaclotide effects not only target constipation but also improve pain and distension in both groups (FC, IBS-C) as seen in clinical trials (benefit of 15-30% over placebo).
Adverse effects
From a practical perspective, the only relevant adverse effect reported was diarrhea, the significance of which should be assessed with the patient. In fact, clinical trials report diarrhea in about 20% of patients on linaclotide, but only 2% of cases are considered as severe. Diarrhea led to drug discontinuation in 4.5% of patients.
Limitations
Linaclotide is not absorbed and does not enter systemic circulation, nor does it affect cytochrome P450. Therefore, while it has not been studied specifically in patients with liver or kidney failure, its use in these patients has no foreseeable limitations. Efficacy and safety are similar in the elderly and in middle-aged adults. Although unlikely, no evidence of teratogenicity exists, hence the drug cannot be recommended during pregnancy. The drug is available in Spain for the treatment of IBS-C, not for FC. It is indicated for FC in other European countries and the USA in half doses.
Practical recommendations
Linaclotide is the drug of choice for patients with constipation and abdominal complaints such as pain and distension when dietary fiber and laxatives have failed (122,123).
27. Usefulness of lubiprostone to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Lubiprostone is a prostaglandin derivative that activates type-2 chloride channels (ClC-2) at the enterocyte's luminal membrane, which increases chloride secretion to the bowel lumen thus enhancing bowel transit. No effects on visceral sensitivity have been described.
Efficacy
Lubiprostone has proven effective to improve constipation symptoms with a NNT of 4 (95% CI: 3-7) (124). Clinical trials in patients with IBS have shown some effects on pain (approximately 7% benefit over placebo) that develop after one month on therapy (124,125).
Adverse effects
Major adverse effects include diarrhea and nausea, the latter occurring in up to 15% of patients in the active group. Although rarely, dyspnea has been described in association with lubiprostone.
Lubiprostone requires no dosage adjustment in patients with kidney failure; while no evidence of liver metabolism exists, the FDA recommends that doses be reduced for patients with Child-Pugh B or C liver disease. Its use is contraindicated during pregnancy, this being a class C drug.
Practical recommendations
Lubiprostone is not available in Europe.
28. Usefulness of anorectal biofeedback to improve: a) constipation; b) abdominal pain; and c) distension
Mechanism of action
Anorectal BFB is a retraining technique indicated for patients with dyssynergic defecation. The physiological activity of the anus and rectum is monitored, and the results are shown to the patient, who is trained on the appropriate maneuvers to correct undesired patterns.
Efficacy
Studies comparing BFB to sham BFB, standard management, laxatives or diazepam (44,126) found that BFB is superior, to variable extents, to all these comparators in improving constipation symptoms. Only one study assessed its effects on abdominal pain, and found significant benefits over laxatives. No study has ever assessed the effects of BFB on abdominal distension.
Adverse effects
None has been described. No limitations exist beforehand for BFB according to patient characteristics, but appropriate willingness and the ability to follow instructions and complete retraining are key factors for success.
Practical recommendation
BFB is the technique of choice for patients with constipation and established pelvic dyssynergia (127).
29. Usefulness of antidepressants to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
The pathways through which these drugs exert their beneficial effects vary according to their class.
Tricyclic antidepressants (TCAs) (amitriptyline): They modulate pain perception at the central nervous system (128).
Selective serotonin reuptake inhibitors (SSRIs) (fluoxetine, paroxetine, citalopram, escitalopram): They decrease visceral sensitivity, improve the sense of well-being, possess anxiolytic properties, and potentiate the effects of other medications, including TCAs (128,129).
Serotonin, norepinephrine reuptake inhibitors (SNRIs) (duloxetine, venlafaxine, desvenlafaxine): They block both serotonin and norepinephrine receptors, thus improving pain control (130).
Efficacy
Regarding the efficacy of TCAs and SSRIs, findings vary according to each individual drug. In a meta-analysis (128), the use of TCAs or SSRIs generally improved distension, pain, and stool consistency in patients with IBS, with a NNT of 4 for both medications (95% CI: 3-6; 4 for TCAs with a 95% CI of 3-8, and 3.5 for SSRIs with a 95% CI of 2-14). However, TCAs should not be used against IBS-C because of their increased constipation effect.
Furthermore, a study (129) of fluoxetine (SSRI) for 16 weeks concluded that this drug, in doses lower than those used for psychiatric disorders, improved all IBS-C symptoms (pain, distension, stool consistency). A randomized, double-blind analysis of paroxetine versus placebo found no significant differences in the primary endpoint (abdominal pain), but did find differences in overall memory and symptom severity (131). Studies supporting citalopram are also available (130).
As regards SNRIs, only duloxetine 60 mg/day has been studied in patients with IBS (132), and proven effective for abdominal pain and stool consistency.
Limitations. Adverse effects (133)
TCAs: They have the greatest number of side effects because of their multiple mechanisms of action (dry mouth, constipation, nausea, vomiting, orthostatic hypotension, etc.). As they markedly enhance constipation, their use in IBS-C (and obviously FC) is advised against. Caution must be exerted in patients with heart conditions, with neurological or urological disorders, and with liver dysfunction, among others.
SSRIs: They are better tolerated than TCAs. Side effects are usually mild but disturbing, and lead to drug discontinuation occasionally. Adverse events include dry mouth, somnolence, reduced libido, anorgasmy, gastrointestinal changes (nausea, diarrhea or constipation), and weight increase.
SNRIs: They are also better tolerated than TCAs. They may induce nausea, somnolence, dizziness, diarrhea, fatigue, constipation, hyperhydrosis, dry mouth, vomiting, decreased appetite, asthenia, and anorexia.
Practical recommendations
The use of antidepressants in doses lower than needed for psychiatric disorders may be indicated for the treatment of persistent distension and pain, and to improve stool consistency in IBS-C. SSRIs are recommended for IBS-C, whereas TCAs should be avoided. Their use should be reserved for patients with persistent symptoms following other therapies (hygienic-dietary measures, laxatives, linaclotide), and for those with an associated psychiatric disorder for which their use has been indicated. When clinically effective, it is advisable that treatment be prolonged for at least 6 months.
Currently, data are insufficient to recommend these drugs in patients with FC, except when indicated to treat psychiatric comorbidity.
30. Usefulness of psychological therapies in patients with IBS to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Several studies have pointed out the association between psychological stress and the worsening of gastrointestinal symptoms in patients with IBS (134-137). These therapies play no role in the management of FC. Psychological therapies may reduce stress, modify the visceral perception threshold, and consequently improve the clinical picture of patients (64) in terms of pain and bowel habit.
Efficacy
A systematic review of psychological therapies including 2,189 patients found a statistically significant effect of these therapies with a NNT of 4 (95% CI: 3-5); however, heterogeneity was significant and quality was poor among the studies involved (90). Furthermore, double-blind studies could not be selected because of the type of treatment. Regarding the 10 types of therapy involved, cognitive-behavioral therapy, hypnotherapy, face-to-face and over-the-telephone multicomponent therapy, and dynamic psychotherapy had proven useful (90).
Hypnosis may modify the visceral perception threshold and provide short-term and long-term clinical improvement (138,139).
Side effects
None has been described.
Limitations
These types of therapy require patient cooperation, as well as time and commitment from both patient and therapist alike. In addition, these therapies are expensive and difficult to be accessed.
Practical recommendations
Some psychological therapies, including cognitive-behavioral therapy and hypnosis, may be useful to manage abdominal pain and reduce stress in patients with IBS.
31. Usefulness of acupuncture in patients with IBS to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects
Mechanism of action
Acupuncture relies on the stimulation of so-called acupuncture or "trigger" points, which are found throughout the body and related to organs and other body components (joints, musculoskeletal bundles, etc.), through the insertion of thin needles into the skin. A number of acupuncture points are related to abdominal pain, diarrhea, and constipation, and may act upon these symptoms when properly stimulated (140).
Efficacy
A meta-analysis including 17 controlled, randomized studies to assess the potential benefits of this technique to improve symptoms and quality of life in patients with IBS found no positive evidence in this regard (141).
Another study that assessed acupuncture having overall symptom improvement as primary endpoint, and the improvement of quality of life and individual symptoms as secondary endpoints, also found no evidence for acupuncture (142).
As of today, no studies have assessed the effects of acupuncture on FC.
Limitations
None have been described.
Practical recommendations
No evidence supports recommending acupuncture to improve symptoms or quality of life in patients with IBS-C or FC.
32. Usefulness of suppositories and enemas as salvage therapy to improve constipation. Adverse effects and special precautions
Enemas and suppositories are essential for the treatment of constipation complicated with fecal impaction, as well as of some cases of obstructive defecation, and to supplement other therapies for severe constipation with significantly impaired bowel transit for the cleansing of the distal colon.
Mechanism of action
Different types of enemas and suppositories are available. All induce rectal distension, thus favoring defecation. Depending on type, enemas may have additional mechanisms of action; for instance, saline enemas drain water towards the colon, whereas phosphate enemas stimulate colonic motility, and mineral oil or emollient enemas lubricate and soften hard feces. Depending also on type, suppositories have different mechanisms of action. Stimulant suppositories containing bisacodyl are available. Glycerin suppositories act locally. The mechanical stimulus of suppository insertion may in itself trigger defecation.
Efficacy
Scientific evidence regarding which type should be used is scarce, but most commonly used enemas include lukewarm water, saline solution (fisioenema) or some sort of osmotic compound (143).
Anal irrigation with Peristeen®, during which some 750 mL of lukewarm water are introduced in the colon, has been successful for patients with neurogenic bowel dysfunction secondary to spinal injury. The number of procedures needed to attain bowel cleansing was reduced, and patient incontinence and quality of life were improved in a randomized trial versus conservative therapy (144). These encouraging results have been subsequently confirmed by an Italian multicenter study, which concluded it may be considered as the treatment of choice for this type of patients (145). Furthermore, this therapy is cost-effective when compared to conservative management (145,146).
Other commonly used enemas include 250 mL sodium phosphate enemas, which exert osmotic effects, and saline solution enemas (fisioenema), which have no side effects and may be acquired over the counter (phosphate enemas are prescription drugs; their side effects are listed in the section below).
As regards suppositories, some act topically to favor rectal ampulla emptying, and some have active ingredients, including bisacodyl, that are dealt with in the section on stimulant laxatives.
Side effects
No significant side effects have been reported for water irrigation using the Peristeen® system.
Regarding phosphate enemas, prolonged use may result in electrolyte imbalance, including hyperphosphatemia, hypocalcemia, and hypernatremia. Therefore, they must be used with caution in patients with a history of electrolyte imbalance including severe renal impairment, in the elderly, in patients with uncontrolled high blood pressure, and in patients with heart failure. Enema abuse may also result in anorectal fibrosis and stenosis from repeat microtrauma (147).
Most commonly reported adverse effects with suppositories include irritation, and anal burning or itching. Given the type of medication involved and its administration route, suppositories have no impact on the concomitant use of other drugs.
Limitations
In some patients, appropriate suppository or enema usage may be challenging (e.g., in patients with motility impairment from spinal injury). However, this difficulty seems smaller with the Peristeen® system.
Practical recommendations
Enemas are useful for constipation complicated with fecal impaction, and as salvage therapy in association with other treatments for severe constipation, although supporting evidence is absent. There is evidence, however, supporting the usefulness of transanal water irrigation using the Peristeen® system in patients with spinal injury, and the system will be likely effective in other severe constipation scenarios.
33. Usefulness of sacral nerve root neurostimulation to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Stimulation of sacral nerves S3-S4 using electrodes that are initially temporarily implanted for about 4 weeks, and then permanently if proven effective. The mechanism of action remains unclear, but the technique seemingly improves rectal sensitivity and colonic contractility, thus improving CTT.
Efficacy
Studies reporting on the efficacy of sacral nerve stimulation focus on patients with slow transit constipation refractory to all treatments, and no controlled studies are available; efficacy is assessed using cross-over designs. A 2007 Cochrane Database of Systematic Reviews meta-analysis found an increase from 2 to 5 in weekly stools, and improved abdominal pain and distension (47). Most relevant is a European multicenter study in 62 patients: the device was permanently implanted in 73% of patients, with sustained constipation, pain, and distension improvement at 28 months of follow-up. However, longer-term benefits remain a concern (148).
Adverse effects
The procedure is not exempt of adverse effects. The multicenter study (148) reported 11 treatment-related serious adverse events; infection, post-implantation pain, mechanical tissue erosion, and electrode migration stand out.
Special precautions include the recommendation to hold back stimulation during prenancy. No safety information is available regarding patients with significant comorbidities.
Practical recommendation
The usefulness of sacral nerve stimulation is controversial, hence should only be considered for patients with intractable SCT FC where dyssynergic defecation has been excluded.
34. Usefulness of surgery to improve: a) constipation; b) abdominal pain; and c) distension. Adverse effects and special precautions
Mechanism of action
Surgery has been suggested for the treatment of severe SCT constipation using resective (colectomy) techniques; the mechanism of action would imply reduced fecal water reabsorption.
Efficacy
No controlled studies of surgery for FC are available, and efficacy must be extrapolated from case report series reviews (48). In this 2011 analysis including 48 studies with 1,443 patients, defecatory frequency improved in 65%, and 88% required no laxatives afterwards. Effectiveness regarding abdominal distension and pain remains unknown.
Adverse effects
According to a case report series review (48), mortality is approximately 0.2%. In addition to immediate complications (ileus: 0-16%, infection: 0-13%, anastomosis dehiscence: 0-22%), other significant delayed complications must be considered (obstruction: 0-74%, incontinence: 0-53%).
Practical recommendation
Surgery should be restricted to exceptional constipation cases with confirmed SCT after excluding intestinal pseudo-obstruction and dyssynergic defecation, and after performing an adequate psychological assessment.
Coordination between levels of care
35. When should a patient with constipation-predominant irritable bowel syndrome or functional constipation be referred to a specialist? Diagnosis and coordination between levels of care
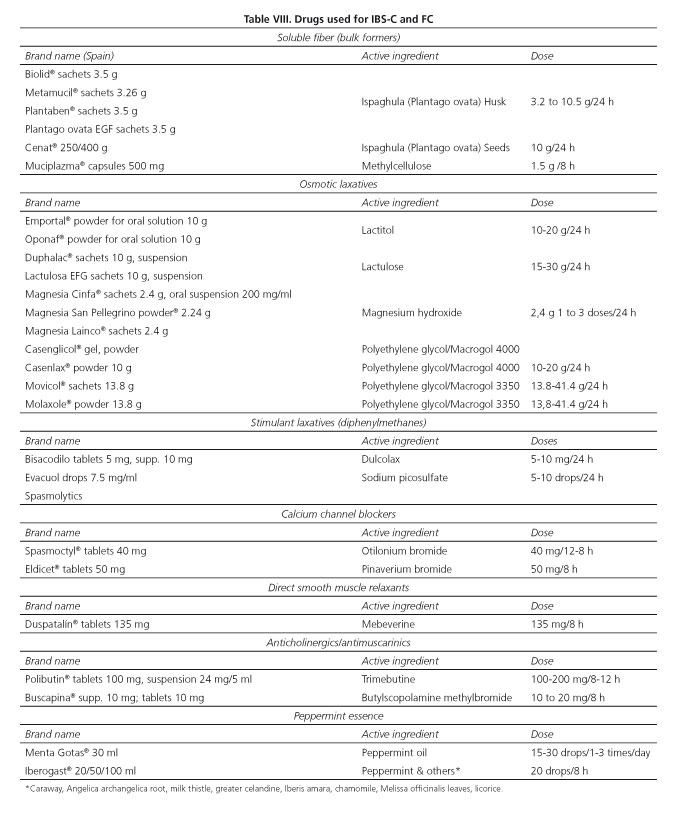
The diagnosis of IBS-C and of FC is well established by the criteria put forward by the Rome's expert panel (Algorithms 1-3, Tables I and II). However, PC clinicians must always be aware of those situations where referral to specialist care should be considered in order to exclude organicity and, on occasion, to optimize the follow-up and treatment of these patients within the frame of integrated, shared care (Algorithms 4 and 5, Tables VIII and IX). Apropriate history taking (including both personal and family history, as well as alarm symptoms and signs) and an attentive physical examination are key factors to reach this end.
Various consensus documents and CPGs establish the reasons that must prompt the ruling out of organic disease, even though accuracy is controversial for some of them (Table VI).
Reasons for referral to a specialist unit also include the presence of persistent symptoms or of symptoms poorly responsive to treatment, severely impaired quality of life, limited access to diagnostic testing, and a doubtful diagnosis (33,37,38,90,149-154).
When should a patient with a firm diagnosis of IBS-C or FC be referred to a gastroenterologist or other specialists?
Currently, each department or health care area may have its own standards of practice according to whether protocols have been set up. Procedure-based standards according to which PC practitioners may access gastroenterologists in order to consult therapy changes or to refer a patient for diagnostic tests or specific therapies exclusively accessible from the specialist care setting would be ideal.
In this respect, patient referral would be appropriate in the following scenarios (see also Table X):
1. Patients not responding or intolerant to basic hygienic-dietary measures, lifestyle changes, and routine laxatives, including rescue therapy with stimulant laxatives (bisacodyl and sodium picosulfate). If virtual access routes are present, serotonin agonists or secretagogues may be prescribed by the PC practitioner, with the consensus of the specialist regarding follow-up. Patients with IBS-C will include non-responders to spasmolytics or antidepressants.
2. Patients with suspected defecatory dysfunction based on anorectal examination.
3. Patients with satisfactory symptom control who experience unexplained worsening. In such cases referral may be made to a gastroenterologist or other specialists according to the associated signs and symptoms (endocrinologist, gynecologist, surgeon, psychiatrist, etc.)
4. Patients who requiere a second opinion from a specialist because of their psychological characteristics, their attitude to symptoms, or their ongoing search for organicity accounting for their complaints.
Conflicts of interest
The authors who sign this Clinical Practice Guideline (CPG) do so on behalf of the Sociedad Española de Patología Digestiva (SEPD), Sociedad Española de Medicina de Familia y Comunitaria (semFYC), Sociedad Española de Médicos de Atención Primaria (SEMERGEN), and Sociedad Española de Médicos Generales y de Familia (SEMG), or as members of the Asociación Española de Gastroenterología (AEG). Neither these scientific societies, nor any of the work group members have any attachments whatsoever with any of the companies that develop drugs for the conditions discussed herein. SEPD, semFYC, SEMERGEN, and SEMG, as well as their members who make up the work group, have no financial interests in the companies that have researched and distribute drugs for these digestive conditions, albeit they maintain, at both, the society and individual levels, a relationship with said companies for education, research, and clinical practice improvement purposes in order to promote digestive health. Finally, SEPD, semFYC, SEMERGEN, and SEMG, as well as the undersigned, declare that the present work was supported by Almirall and Allergan but received no external influences, and that no third parties took part in the discussions and development of the Guideline, or had access to the final manuscript before its publication in the Revista Española de Enfermedades Digestivas and the official journals of the societies involved.
Acknowledgements
The authors are grateful for the cooperation of the four scientific societies involved, for the help received from Marién Castillo-Sánchez, and for the contributions of Joaquín León-Molina as literature manager and José María Tenías as methodological support. They are also particularly grateful for the suggestions and comments received from the following external reviewers: Agustín Balboa-Rodríguez, R. Manuel Devesa-Muñiz, Higinio Flores-Tirado, Juan Manuel Mendive-Arbeloa, Xavier Puigdengolas-Armengol, Mercedes Ricote-Belinchón, Cecilio Santan-der-Vaquero, Jordi Serra-Pueyo, Carlos A. Siljeström-Laredo, and María José Soria-de-la-Cruz.
Note on methodology: This CPG has been developed between January 2015 and December 2015 by a work group made up by experts selected from SEPD, semFYC, SEMERGEN, and SEMG. The Guidelines were revised from December 2015 to April 2016, and made consistent with the Rome IV criteria in May 2016.
For a detailed description of the methodological process undertaken during the development of the present CPG, see: http://www.sepd.es/file/GPC_SII_E_EF_Metodologia.pdf
Additionally to this full version of the CPG, a two-part version is being published, in coordination, by each channel of expression of semFYC, SEMERGEN and SEMG (in press).
References
1. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130(5):1480-91. PMID: 16678561. DOI: 10.1053/j.gastro.2005.11.061. [ Links ]
2. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393-407. [ Links ]
3. Rey E, Balboa A, Mearin F. Chronic constipation, irritable bowel syndrome with constipation and constipation with pain/discomfort: Similarities and differences. American Journal of Gastroenterology 2014;109(6):876-84. PMID: 24589666. DOI: 10.1038/ajg.2014.18. [ Links ]
4. Wong RK, Palsson OS, Turner MJ, et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. American Journal of Gastroenterology 2010;105(10):2228-34. PMID: 20502449. DOI: 10.1038/ajg.2010.200. [ Links ]
5. Bharucha AE, Locke GR, Zinsmeister AR, et al. Differences between painless and painful constipation among community women. American Journal of Gastroenterology 2006;101(3):604-12. PMID: 16464225. DOI: 10.1111/j.1572-0241.2006.00435.x. [ Links ]
6. Kamm MA, Muller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: A randomized, double-blind, placebo-controlled multinational study. American Journal of Gastroenterology 2005;100(2):362-72. PMID: 15667494. DOI: 10.1111/j.1572-0241.2005.40749.x. [ Links ]
7. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Alimentary Pharmacology & Therapeutics 2002;16(11):1877-88. PMID: 12390096. DOI: 10.1046/j.1365-2036.2002.01372.x. [ Links ]
8. Johanson JF, Morton D, Geenen J, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. American Journal of Gastroenterology 2008;103(1):170-7. PMID: 17916109. DOI: 10.1111/j.1572-0241.2007.01524.x. [ Links ]
9. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: Lubiprostone in patients with constipation-associated irritable bowel syndrome - Results of two randomized, placebo-controlled studies. Alimentary Pharmacology & Therapeutics 2009;29(3):329-41. PMID: 19006537. DOI: 10.1111/j.1365-2036.2008.03881.x. [ Links ]
10. Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. New England Journal of Medicine 2011;365(6):527-36. PMID: 21830967. DOI: 10.1056/NEJ-Moa1010863. [ Links ]
11. Johnston JM, Kurtz CB, MacDougall JE, et al. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology 2010;139(6):1877-U139. PMID: 20801122. DOI: 10.1053/j.gastro.2010.08.041. [ Links ]
12. Vandvik PO, Wilhelmsen I, Ihlebaek C, et al. Comorbidity of irritable bowel syndrome in general practice: A striking feature with clinical implications. Alimentary Pharmacology & Therapeutics 2004;20(10):1195-203. PMID: 15569123. DOI: 10.1111/j.1365-2036.2004.02250.x. [ Links ]
13. Whitehead WE, Palsson OS, Levy RR, et al. Comorbidity in irritable bowel syndrome. American Journal of Gastroenterology 2007;102(12):2767-76. PMID: 17900326. DOI: 10.1111/j.1572-0241.2007.01540.x. [ Links ]
14. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology 1997;32(9):920-4. PMID: 9299672. DOI: 10.3109/00365529709011203. [ Links ]
15. Bharucha AE, Wald AM. Anorectal disorders. American Journal of Gastroenterology 2010;105(4):786-94. PMID: 20372131. DOI: 10.1038/ajg.2010.70. [ Links ]
16. Johanson JF, Kralstein J. Chronic constipation: A survey of the patient perspective. Alimentary Pharmacology & Therapeutics 2007;25(5):599-608. PMID: 17305761. DOI: 10.1111/j.1365-2036.2006.03238.x. [ Links ]
17. Posserud I, Syrous A, Lindstrom L, et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 2007;133(4):1113-23. PMID: 17919487. DOI: 10.1053/j.gastro.2007.07.024. [ Links ]
18. Ng C, Danta M, Kellow J, et al. Attenuation of the colorectal tonic reflex in female patients with irritable bowel syndrome. American Journal of Physiology-Gastrointestinal and Liver Physiology 2005;289(3):G489-G94. PMID: 15905412. DOI: 10.1152/ajpgi.00527.2004. [ Links ]
19. Burgell RE, Scott SM. Rectal hyposensitivity. Journal of Neurogastroenterology and Motility 2012;18(4):373-84. PMID: 23105997. DOI: 10.5056/jnm.2012.18.4.373. [ Links ]
20. El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: A systematic review. Alimentary Pharmacology & Therapeutics 2002;16(6):1171-85. PMID: 12030961. DOI: 10.1046/j.1365-2036.2002.01290.x. [ Links ]
21. Mearin F, Perello A, Perona M. Quality of life in patients with irritable bowel syndrome. Gastroenterología y Hepatología 2004;27(Suppl. 3):24-31. DOI: 10.1157/13058927. [ Links ]
22. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part II: Lower gastrointestinal diseases. Gastroenterology 2009;136(3):741-54. PMID: 19166855. DOI: 10.1053/j.gastro.2009.01.015. [ Links ]
23. Tack J, Stanghellini V, Mearin F, et al. Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries: A 12-month observational study. En prensa 2016. [ Links ]
24. Sun SX, DiBonaventura M, Purayidathil FW, et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: An analysis of the National Health and Wellness Survey. Digestive Diseases and Sciences 2011;56(9):2688-95. PMID: 21380761. DOI: 10.1007/s10620-011-1639-5. [ Links ]
25. Dennison C, Prasad M, Lloyd A, et al. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005;23(5):461-76. PMID: 15896098. DOI: 10.2165/00019053-200523050-00006. [ Links ]
26. Belsey J, Greenfield S, Candy D, et al. M. Systematic review: Impact of constipation on quality of life in adults and children. Alimentary Pharmacology & Therapeutics 2010;31(9):938-49. PMID: 20180788. DOI: 10.1111/j.1365-2036.2010.04273.x. [ Links ]
27. Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: A Rome Foundation working team report. American Journal of Gastroenterology 2011;106(10):1749-59. PMID: 21747417. DOI: 10.1038/ajg.2011.201. [ Links ]
28. Mearin F, Lacy BE. Diagnostic criteria in IBS: Useful or not? Neurogastroenterology and Motility 2012;24(9):791-801. PMID: 22908861. DOI: 10.1111/j.1365-2982.2012.01992.x. [ Links ]
29. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacology & Therapeutics 1997;11(2):395-402. PMID: 9146781. DOI: 10.1046/j.1365-2036.1997.142318000.x. [ Links ]
30. Almansa C, García R, Barceló M, et al. Translation, cultural adaptation and validation of a Spanish version of the Irritable Bowel Syndrome Severity Score. Revista Española De Enfermedades Digestivas 2011;103(12):612-8. PMID: 22217344. DOI: 10.4321/S1130-01082011001200002. [ Links ]
31. Brandt LJ, Schoenfeld P, Prather CM, et al. Evidence-based position statement on the management of chronic constipation in North America. American Journal of Gastroenterology 2005;100(1). DOI: 10.1111/j.1572-0241.2005.50613_2.x. [ Links ]
32. Bharucha AE, Wald A, Enck P, et al. Functional anorectal disorders. Gastroenterology 2006;130(5):1510-8. PMID: 16678564. DOI: 10.1053/j.gastro.2005.11.064. [ Links ]
33. Bharucha AE, Dorn SD, Lembo A, el al. American Gastroenterological A. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144(1):211-7. PMID: 23261064. DOI: 10.1053/j.gastro.2012.10.029. [ Links ]
34. Wald A, Bharucha AE, Cosman BC, et al. ACG Clinical Guideline: Management of benign anorectal disorders. American Journal of Gastroenterology 2014;109(8):1141-57. PMID: 25022811. DOI: 10.1038/ajg.2014.190. [ Links ]
35. Rao SSC, Meduri K. What is necessary to diagnose constipation? Best Practice & Research in Clinical Gastroenterology 2011;25(1):127-40. PMID: 21382584. DOI: 10.1016/j.bpg.2010.11.001. [ Links ]
36. Videlock EJ, Lembo A, Cremonini F. Diagnostic testing for dyssynergic defecation in chronic constipation: Meta-analysis. Neurogastroenterology and Motility 2013;25(6). PMID: 23644388. DOI: 10.1111/nmo.12096. [ Links ]
37. Mínguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology 2004;126(1):57-62. PMID: 14699488. DOI: 10.1053/j.gastro.2003.10.044. [ Links ]
38. Tack J, Mueller-Lissner S, Stanghellini V, et al. Diagnosis and treatment of chronic constipation - An European perspective. Neurogastroenterology and Motility 2011;23(8):697-710. PMID: 21605282. DOI: 10.1111/j.1365-2982.2011.01709.x. [ Links ]
39. Spanish Group for the Study of Digestive Motility. Measurement of colonic transit time (total and segmental) with radiopaque markers. National reference values obtained in 192 healthy subjects. Gastroenterología y hepatología 1998;21(2):71-5. PMID: 9549181. [ Links ]
40. Bonapace ES, Maurer AH, Davidoff S, et al. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. American Journal of Gastroenterology 2000;95(10):2838-47. PMID: 11051357. DOI: 10.1111/j.1572-0241.2000.03195.x. [ Links ]
41. Burton DD, Camilleri M, Mullan BP, et al. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. Journal of Nuclear Medicine 1997;38(11):1807-10. PMID: 9374360. [ Links ]
42. Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: Prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterology and Motility 2010;22(8):874-E233. PMID: 20465593. DOI: 10.1111/j.1365-2982.2010.01517.x [ Links ]
43. Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129(1):86-97. PMID: 16012938. DOI: 10.1053/j.gastro.2005.05.015. [ Links ]
44. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130(3):657-64. PMID: 16530506. DOI: 10.1053/j.gastro.2005.11.014. [ Links ]
45. Rao SSC, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clinical Gastroenterology and Hepatology 2007;5(3):331-8. PMID: 17368232. DOI: 10.1016/j.cgh.2006.12.023. [ Links ]
46. Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Diseases of the Colon & Rectum 2007;50(4):428-41. PMID: 17294322. DOI: 10.1007/s10350-006-0814-9. [ Links ]
47. Kamm MA, Dudding TC, Melenhorst J, et al. Sacral nerve stimulation for intractable constipation. Gut 2010;59(3):333-40. PMID: 20207638. DOI: 10.1136/gut.2009.187989. [ Links ]
48. Arebi N, Kalli T, Howson W, et al. Systematic review of abdominal surgery for chronic idiopathic constipation. Colorectal Disease 2011;13(12):1335-43. PMID: 20969711. DOI: 10.1111/j.1463-1318.2010.02465.x. [ Links ]
49. Bove A, Bellini M, Battaglia E, et al. Consensus statement AIGO/SICCR diagnosis and treatment of chronic constipation and obstructed defecation (Part II: Treatment). World Journal of Gastroenterology 2012;18(36):4994-5013. PMID: 23049207. DOI: 10.3748/wjg.v18.i36.4994. [ Links ]
50. Muller-Lissner SA, Kamm MA, Scarpignato C, et al. Myths and misconceptions about chronic constipation. American Journal of Gastroenterology 2005;100(1):232-42. PMID: 15654804. DOI: 10.1111/j.1572-0241.2005.40885.x. [ Links ]
51. Moylan S, Armstrong J, Díaz-Saldano D, et al. Are abdominal x-rays a reliable way to assess for constipation? Journal of Urology 2010;184(4):1692-7. PMID: 20728159. DOI: 10.1016/j.juro.2010.05.054. [ Links ]
52. Pensabene L, Buonomo C, Fishman L, et al. Lack of utility of abdominal x-rays in the evaluation of children with constipation: Comparison of different scoring methods. Journal of Pediatric Gastroenterology and Nutrition 2010;51(2):155-9. PMID: 20453675. DOI: 10.1097/MPG.0b013e3181cb4309. [ Links ]
53. Patriquin H, Martelli H, Devroede G. Barium enema in chronic constipation - Is it meaningful. Gastroenterology 1978;75(4):619-22. PMID: 710831. [ Links ]
54. Pepin C, Ladabaum U. The yield of lower endoscopy in patients with constipation: Survey of a university hospital, a public county hospital, and a Veterans Administration medical center. Gastrointestinal Endoscopy 2002;56(3):325-32. PMID: 12196767. DOI: 10.1067/mge.2002.126882. [ Links ]
55. Gupta M, Holub J, Knigge K, et al. Constipation is not associated with an increased rate of findings on colonoscopy: Results from a national endoscopy consortium. Endoscopy 2010;42(3):208-12. PMID: 20101567. DOI: 10.1055/s-0029-1243843. [ Links ]
56. Mertz H, Naliboff B, Mayer EA. Symptoms and physiology in severe chronic constipation. American Journal of Gastroenterology 1999;94(1):131-8. PMID: 9934743. DOI: 10.1111/j.1572-0241.1999.00783.x. [ Links ]
57. Eltringham MT, Khan U, Bain IM, et al. Functional defecation disorder as a clinical subgroup of chronic constipation: Analysis of symptoms and physiological parameters. Scandinavian Journal of Gastroenterology 2008;43(3):262-9. PMID: 18266173. DOI: 10.1080/00365520701686210. [ Links ]
58. Koch A, Voderholzer WA, Klauser AG, et al. Symptoms in chronic constipation. Diseases of the Colon & Rectum 1997;40(8):902-6. PMID: 9269805. DOI: 10.1007/BF02051196. [ Links ]
59. Orkin BA, Sinykin SB, Lloyd PC. The Digital Rectal Examination Scoring System (DRESS). Diseases of the Colon & Rectum 2010;53(12):1656-60. PMID: 21178861. DOI: 10.1007/DCR.0b013e3181f23c85. [ Links ]
60. Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association 2010;8(11):955-60. PMID: 20656061. DOI: 10.1016/j.cgh.2010.06.031. [ Links ]
61. Glia A, Lindberg G, Nilsson LH, et al. Clinical value of symptom assessment in patients with constipation. Diseases of the Colon & Rectum 1999;42(11):1401-8. PMID: 10566527. DOI: 10.1007/BF02235036. [ Links ]
62. Saad RJ, Rao SSC, Koch KL, et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. American Journal of Gastroenterology 2010;105(2):403-11. PMID: 19888202. DOI: 10.1038/ajg.2009.612. [ Links ]
63. Lam TJ, Felt-Bersma RJF. Clinical examination remains more important than anorectal function tests to identify treatable conditions in women with constipation. International Urogynecology Journal 2013;24(1):67-72. PMID: 22618205. DOI: 10.1007/s00192-012-1796-x. [ Links ]
64. Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World Journal of Gastroenterology 2014;20(22):6759-73. PMID: 24944467. DOI: 10.3748/wjg.v20.i22.6759. [ Links ]
65. De Schryver AM, Keulemans YC, Peters HP, et al. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scandinavian Journal of Gastroenterology 2005;40(4):422-9. PMID: 16028436. DOI: 10.1080/00365520510011641. [ Links ]
66. Villoria A, Serra J, Azpiroz F, et al. Physical activity and intestinal gas clearance in patients with bloating. American Journal of Gastroenterology 2006;101(11):2552-7. PMID: 17029608. DOI: 10.1111/j.1572-0241.2006.00873.x. [ Links ]
67. Dainese R, Serra J, Azpiroz F, et al. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. American Journal of Medicine 2004;116(8):536-9. PMID: 15063815. DOI: 10.1016/j.amjmed.2003.12.018. [ Links ]
68. Johannesson E, Ringstrom G, Abrahamsson H, et al. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World Journal of Gastroenterology 2015;21(2):600-8. PMID: 25593485. DOI: 10.3748/wjg.v21.i2.600. [ Links ]
69. Johannesson E, Simren M, Strid H, et al. Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. American Journal of Gastroenterology 2011;106(5):915-22. PMID: 21206488. DOI: 10.1038/ajg.2010.480. [ Links ]
70. Ternent CA, Amir LB, Morin NA, et al. Practice parameters for the evaluation and management of constipation. Diseases of the Colon & Rectum 2007;50(12):2013-22. PMID: 17665250. DOI: 10.1007/s10350-007-9000-y. [ Links ]
71. Remes Troche JM, Gómez Escudero O, Icaza Chávez ME, et al. Guidelines for diagnosis and treatment of constipation in Mexico. C) Medical and surgical treatment. Revista de Gastroenterología de México 2011;76(2):141-54. PMID: 21724490. [ Links ]
72. Gallegos-Orozco JF, Foxx-Orenstein AE, Sterler SM, et al. Chronic constipation in the elderly. American Journal of Gastroenterology 2012;107(1):18-25. PMID: 21989145. DOI: 10.1038/ajg.2011.349. [ Links ]
73. Anti M, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepato-Gastroenterology 1998;45(21):727-32. PMID: 9684123. [ Links ]
74. Lever E, Cole J, Scott SM, et al. Systematic review: The effect of prunes on gastrointestinal function. Alimentary Pharmacology & Therapeutics 2014;40(7):750-8. PMID: 25109788. DOI: 10.1111/apt.12913. [ Links ]
75. Bijkerk CJ, Muris JWM, Knottnerus JA, et al. Systematic review: The role of different types of fibre in the treatment of irritable bowel syndrome. Alimentary Pharmacology & Therapeutics 2004;19(3):245-51. PMID: 14984370. DOI: 10.1111/j.0269-2813.2004.01862.x. [ Links ]
76. Ford AC, Talley NJ, Spiegel BMR, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: Systematic review and meta-analysis. British Medical Journal 2008;337:PMID: 19008265. DOI: 10.1136/bmj.a2313. [ Links ]
77. Carroccio A, Mansueto P, Iacono G, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. American Journal of Gastroenterology 2012;107(12):1898-906. PMID: 22825366. DOI: 10.1038/ajg.2012.236. [ Links ]
78. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013;145(2):320-+. PMID: 23648697. DOI: 10.1053/j.gastro.2013.04.051. [ Links ]
79. Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An Evidence-based position statement on the management of irritable bowel syndrome. American Journal of Gastroenterology 2009;104:S1-S36. PMID: 19521341. [ Links ]
80. Staudacher HM, Lomer MCE, Anderson JL, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. Journal of Nutrition 2012;142(8):1510-8. PMID: 22739368. DOI: 10.3945/jn.112.159285. [ Links ]
81. Boehn L, Stoersrud S, Liljebo TM, et al. A randomized, controlled trial comparing a diet low in FODMAPs with traditional dietary advice in patients with IBS. Gastroenterology 2015;148(4):S654-S. PMID: 26255043. [ Links ]
82. Stephen AM, Cummings JH. Mechanism of action of dietary fiber in the human-colon. Nature 1980;284(5753):283-4. PMID: 7360261. [ Links ]
83. Rao SSC, Yu S, Fedewa A. Systematic review: Dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Alimentary Pharmacology & Therapeutics 2015;41(12):1256-70. PMID: 25903636. DOI: 10.1111/apt.13167 [ Links ]
84. Yang J, Wang H-P, Zhou L, et al. Effect of dietary fiber on constipation: A meta analysis. World Journal of Gastroenterology 2012;18(48):7378-83. PMID: 23326148. DOI: 10.3748/wjg.v18.i48.7378. [ Links ]
85. Suares NC, Ford AC. Systematic review: The effects of fibre in the management of chronic idiopathic constipation. Alimentary Pharmacology & Therapeutics 2011;33(8):895-901. PMID: 21332763. DOI: 10.1111/j.1365-2036.2011.04602.x. [ Links ]
86. Francis CY, Whorwell PJ. Bran and irritable-bowel-syndrome - Time for reappraisal. Lancet 1994;344(8914):39-40. PMID: 7912305. [ Links ]
87. Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. American Journal of Gastroenterology 1997;92(1):95-8. PMID: 8995945. [ Links ]
88. Awad RA, Camacho S. A randomized, double-blind, placebo-controlled trial of polyethylene glycol effects on fasting and postprandial rectal sensitivity and symptoms in hypersensitive constipation-predominant irritable bowel syndrome. Colorectal Disease 2010;12(11):1131-8. PMID: 19575740. DOI: 10.1111/j.1463-1318.2009.01990.x. [ Links ]
89. Chapman RW, Stanghellini V, Geraint M, et al. Randomized clinical trial: Macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. American Journal of Gastroenterology 2013;108(9):1508-15. PMID: 23835436. DOI: 10.1038/ajg.2013.197. [ Links ]
90. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. American Journal of Gastroenterology 2014;109:S2-S26. PMID: 25091148. DOI: 10.1038/ajg.2014.187. [ Links ]
91. Lee-Robichaud H, Thomas K, Morgan J, et al. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database of Systematic Reviews 2010(7). PMID: 20614462. [ Links ]
92. Hookway C, Buckner S, Crosland P, et al. Irritable bowel syndrome in adults in primary care: Summary of updated NICE guidance. Bmj-British Medical Journal 2015;350. PMID: 25716701. DOI: 10.1136/bmj.h701. [ Links ]
93. Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clinical Gastroenterology and Hepatology 2011;9(7):577-83. PMID: 21440672. DOI: 10.1016/j.cgh.2011.03.026. [ Links ]
94. Mueller-Lissner S, Kamm MA, Wald A, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. American Journal of Gastroenterology 2010;105(4):897-903. PMID: 20179697. DOI: 10.1038/ajg.2010.41. [ Links ]
95. Bernet MF, Brassart D, Neeser JR, et al. Lactobacillus-acidophilus la-1 binds to cultured human intestinal-cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 1994;35(4):483-9. PMID: 8174985. DOI: 10.1136/gut.35.4.483. [ Links ]
96. Wang XD, Soltesz V, Molin G, et al. The role of oral-administration of oatmeal fermented by lactobacillus-reuteri r2lc on bacterial translocation after acute liver-failure induced by subtotal liver resection in the rat. Scandinavian Journal of Gastroenterology 1995;30(2):180-5. PMID: 7732342. DOI: 10.3109/00365529509093259. [ Links ]
97. Kaila M, Isolauri E, Soppi E, et al. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human lactobacillus strain. Pediatric Research 1992;32(2):141-4. PMID: 1324462. DOI: 10.1203/00006450-199208000-00002. [ Links ]
98. O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005;128(3):541-51. PMID: 15765388. DOI: 10.1053/j.gastro.2004.11.050. [ Links ]
99. Ford AC, Quigley EMM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. American Journal of Gastroenterology 2014;109(10):1547-61. PMID: 25070051. DOI: 10.1038/ajg.2014.202. [ Links ]
100. Koebnick C, Wagner I, Leitzmann P, et al. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Canadian Journal of Gastroenterology 2003;17(11):655-9. PMID: 14631461. DOI: 10.1155/2003/654907. [ Links ]
101. Pimentel M, Chang C, Chua KS, et al. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Digestive Diseases and Sciences 2014;59(6):1278-85. PMID: 24788320. DOI: 10.1007/s10620-014-3157-8. [ Links ]
102. Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (Rifaximin) on the symptoms of the irritable bowel syndrome - A randomized trial. Annals of Internal Medicine 2006;145(8):557-63. PMID: 17043337. DOI: 10.7326/0003-4819-145-8-200610170-00004. [ Links ]
103. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. New England Journal of Medicine 2011;364(1):22-32. PMID: 21208106. DOI: 10.1056/NEJMoa1004409. [ Links ]
104. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: A double-blind, randomized, placebo-controlled study. American Journal of Gastroenterology 2003;98(2):412-9. PMID: 12591062. DOI: 10.1111/j.1572-0241.2003.07234.x. [ Links ]
105. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome - art. no. CD003460.pub2. Cochrane Database of Systematic Reviews 2005(2). PMID: 15846668. [ Links ]
106. Clave P, Acalovschi M, Triantafillidis JK, et al. Randomised clinical trial: Otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Alimentary Pharmacology & Therapeutics 2011;34(4):432-42. PMID: 21679214. [ Links ]
107. Capanni M, Surrenti E, Biagini M, et al. Efficacy of peppermint oil in the treatment of irritable bowel syndrome: A randomized, controlled trial. Gazzetta Medica Italiana 2005(164):119-26. [ Links ]
108. Cappello G, Spezzaferro M, Grossi L, et al. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Digestive and Liver Disease 2007;39(6):530-6. PMID: 17420159. DOI: 10.1016/j.dld.2007.02.006. [ Links ]
109. Lech Y, Olesen KM, Hey H, et al. Treatment of irritable bowel syndrome with peppermint oil. A double-blind study with a placebo. Ugeskrift for laeger 1988;150(40):2388-9. PMID: 3061106. [ Links ]
110. Liu JH, Chen GH, Yeh HZ, et al. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: A prospective, randomized trial. Journal of Gastroenterology 1997;32(6):765-8. PMID: 9430014. DOI: 10.1007/BF02936952. [ Links ]
111. Merat S, Khalili S, Mostajabi P, et al. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Digestive Diseases and Sciences 2010;55(5):1385-90. PMID: 19507027. DOI: 10.1007/s10620-009-0854-9. [ Links ]
112. Kim DY, Camilleri M. Serotonin: A mediator of the brain-gut connection. American Journal of Gastroenterology 2000;95(10):2698-709. PMID: 11051338. DOI: 10.1111/j.1572-0241.2000.03177.x. [ Links ]
113. Bassotti G, Blandizzi C. Understanding and treating refractory constipation. World Journal of Gastrointestinal Pharmacology and Therapeutics. 2014;5(2):77-85. PMID: 24868488. [ Links ]
114. Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. New England Journal of Medicine 2008;358(22):2344-54. PMID: 18509121. DOI: 10.1056/NEJMoa0800670. [ Links ]
115. Tack J, Van Outryve M, Beyens G, et al. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 2009;58(3):357-65. PMID: 18987031. DOI: 10.1136/gut.2008.162404. [ Links ]
116. Quigley EMM, Vandeplassche L, Kerstens R, et al. Clinical trial: The efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation - A 12-week, randomized, double-blind, placebo-controlled study. Alimentary Pharmacology & Therapeutics 2009;29(3):315-28. PMID: 19035970. DOI: 10.1111/j.1365-2036.2008.03884.x. [ Links ]
117. Shin A, Camilleri M, Kolar G, et al. Systematic review with meta-analysis: Highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Alimentary Pharmacology & Therapeutics 2014;39(3):239-53. PMID: 24308797. DOI: 10.1111/apt.12571. [ Links ]
118. Camilleri M, Beyens G, Kerstens R, et al. Safety assessment of prucalopride in elderly patients with constipation: A double-blind, placebo-controlled study. Neurogastroenterology and Motility 2009;21(12). PMID: 19751247. DOI: 10.1111/j.1365-2982.2009.01398.x. [ Links ]
119. Emmanuel AV, Kamm MA, Roy AJ, et al. Randomised clinical trial: The efficacy of prucalopride in patients with chronic intestinal pseudo-obstruction - A double-blind, placebo-controlled, cross-over, multiple n = 1 study. Alimentary Pharmacology & Therapeutics 2012;35(1):48-55. PMID: 22061077. DOI: 10.1111/j.1365-2036.2011.04907.x. [ Links ]
120. Busby RW, Bryant AP, Bartolini WP, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. European Journal of Pharmacology 2010;649(1-3):328-35. PMID: 20863829. DOI: 10.1016/j.ejphar.2010.09.019. [ Links ]
121. Eutamene H, Bradesi S, Larauche M, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterology and Motility 2010;22(3). PMID: 19706070. DOI: 10.1111/j.1365-2982.2009.01385.x. [ Links ]
122. Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: A meta-analysis. Clinical Gastroenterology and Hepatology 2013;11(9):1084-U82. PMID: 23421551. DOI: 10.1016/j.cgh.2013.04.032. [ Links ]
123. Atluri DK, Chandar AK, Bharucha AE, et al. Effect of linaclotide in irritable bowel syndrome with constipation (IBS-C): A systematic review and metaanalysis. Neurogastroenterology and Motility 2014;26(4):499-509. PMID: 24351035. DOI: 10.1111/nmo.12292. [ Links ]
124. Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: Systematic review and meta-analysis. Gut 2011;60(2):209-18. PMID: 21205879. DOI: 10.1136/gut.2010.227132. [ Links ]
125. Wilson N, Schey R. Lubiprostone in constipation: Clinical evidence and place in therapy. Therapeutic advances in chronic disease 2015;6(2):40-50. PMID: 25729555. DOI: 10.1177/2040622314567678. [ Links ]
126. Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database of Systematic Reviews 2014(3). PMID: 24668156. [ Links ]
127. Rao SSC, Benninga MA, Bharucha AE, et al. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil 2015;27(5):594-609. PMID: 25828100. DOI: 10.1111/nmo.12520. [ Links ]
128. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: Systematic review and meta-analysis. Gut 2009;58(3):367-78. PMID: 19001059. DOI: 10.1136/gut.2008.163162. [ Links ]
129. Vahedi H, Merat S, Rashidioon A, et al. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: A double-blind randomized-controlled study. Alimentary Pharmacology & Therapeutics 2005;22(5):381-5. PMID: 16128675. DOI: 10.1111/j.1365-2036.2005.02566.x. [ Links ]
130. Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55(8):1095-103. PMID: 16401691. [ Links ]
131. Masand PS, Pae C-U, Krulewicz S, et al. A double-blind, randomized, placebo-controlled trial of paroxetine controlled-release in irritable bowel syndrome. Psychosomatics 2009;50(1):78-86. PMID: 19213976. DOI: 10.1176/appi.psy.50.1.78. [ Links ]
132. Brennan BP, Fogarty KV, Roberts JL, et al. Duloxetine in the treatment of irritable bowel syndrome: An open-label pilot study. Human Psychopharmacology-Clinical and Experimental 2009;24(5):423-8. PMID: 19548294. DOI: 10.1002/hup.1038. [ Links ]
133. Colonna L. Antidepresseurs. In: Flammarion MS, editor. Vassilis Kapsambelis D Ginestet: Thérapeutique médicamenteuse des troubles psychiatriques de l'adulte. París; 1996. p. 29-41. [ Links ]
134. Liu JP, Yang M, Lix YX, et al. Herbal medicines for treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2006(1). PMID: 16437473. [ Links ]
135. Park HJ, Jarrett M, Cain K, et al. Psychological distress and GI symptoms are related to severity of bloating in women with irritable bowel syndrome. Research in Nursing & Health 2008;31(2):98-107. PMID: 18181134. DOI: 10.1002/nur.20237. [ Links ]
136. Blanchard EB, Lackner JM, Jaccard J, et al. The role of stress in symptom exacerbation among IBS patients. Journal of Psychosomatic Research 2008;64(2):119-28. PMID: 18222125. DOI: 10.1016/j.jpsychores.2007.10.010. [ Links ]
137. Choung RS, Locke GR 3rd, Zinsmeister AR, et al. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: A psychological component is the rule. American Journal of Gastroenterology 2009;104(7):1772-9. PMID: 19491833. DOI: 10.1038/ajg.2009.239. [ Links ]
138. Lindfors P, Unge P, Arvidsson P, et al. Effects of gut-directed hypnotherapy on IBS in different clinical settings-results from two randomized, controlled trials. American Journal of Gastroenterology 2012;107(2):276-85. PMID: 21971535. DOI: 10.1038/ajg.2011.340. [ Links ]
139. Webb AN, Kukuruzovic RH, Catto-Smith AG, et al. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2007(4). PMID: 17943840. [ Links ]
140. Dorsher P, Fleckenstein J. Puntos gatillo y puntos de acupuntura clásica. Parte 2: Correspondencias clínicas en el tratamiento del dolor y las disfunciones somatoviscerales. Revista Internacional de Acupuntura 2009;36(2). DOI: 10.1016/S1887-8369(09)71576-5. [ Links ]
141. Manheimer E, Wieland LS, Cheng K, et al. Acupuncture for irritable bowel syndrome: Systematic review and meta-analysis. American Journal of Gastroenterology 2012;107(6):835-47. PMID: 22488079. DOI: 10.1038/ajg.2012.66. [ Links ]
142. Lembo AJ, Conboy L, Kelley JM, et al. A treatment trial of acupuncture in ibs patients. American Journal of Gastroenterology 2009;104(6):1489-97. PMID: 19455132. DOI: 10.1038/ajg.2009.156. [ Links ]
143. Hussain ZH, Whitehead DA, Lacy BE. Fecal impaction. Current Gastroenterology Reports 2014;16(9):404-. PMID: 25119877. DOI: 10.1007/s11894-014-0404-2. [ Links ]
144. Christensen P, Bazzocchi G, Coggrave M, et al. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 2006;131(3):738-47. PMID: 16952543. DOI: 10.1053/j.gastro. 2006.06.004. [ Links ]
145. Del Popolo G, Mosiello G, Pilati C, et al. Treatment of neurogenic bowel dysfunction using transanal irrigation: A multicenter Italian study. Spinal Cord 2008;46(7):517-22. PMID: 18317488. DOI: 10. 1038/sj.sc.3102167. [ Links ]
146. Christensen P, Andreasen J, Ehlers L. Cost-effectiveness of transanal irrigation versus conservative bowel management for spinal cord injury patients. Spinal Cord 2009;47(2):138-43. PMID: 18679401. DOI: 10.1038/sc.2008.98. [ Links ]
147. Podzemny V, Pescatori LC, Pescatori M. Management of obstructed defecation. World Journal of Gastroenterology 2015;21(4):1053-60. PMID: 25632177. DOI: 10.3748/wjg.v21.i4.1053. [ Links ]
148. Mowatt G, Glazener C, Jarrett M. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database of Systematic Reviews 2007(3). PMID: 17636759. [ Links ]
149. Mínguez M, Mora F, Mas P, et al. Guía práctica de actuación diagnóstico-terapéutica en estreñimiento crónico. Valencia: Fundación Española de Aparato Digestivo; 2013. [ Links ]
150. Fukudo S, Kaneko H, Akiho H, et al. Evidence-based clinical practice guidelines for irritable bowel syndrome. Journal of Gastroenterology 2015;50(1):11-30. PMID: 25500976. DOI: 10.1007/s00535-014-1017-0. [ Links ]
151. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. Jama-Journal of the American Medical Association 2015;313(9):949-58. PMID: 25734736. DOI: 10.1001/jama.2015.0954. [ Links ]
152. Grupo de trabajo de la Guía de Práctica Clínica sobre el síndrome del intestino irritable. Manejo del paciente con síndrome del intestino irritable. Barcelona: Asociación Española de Gastroenterología, Sociedad Española de Familia y Comunitaria y Centro Cochrane Iberoamericano; 2005. [ Links ]
153. Serra J, Mascort-Roca J, Marzo-Castillejo M, et al. Guía de práctica clínica sobre el manejo del estreñimiento crónico en el paciente adulto. Parte 1: Definición, etiología y manifestaciones clínicas. Gastroenterol Hepatol 2016; en prensa. [ Links ]
154. Serra J, Mascort-Roca J, Marzo-Castillejo M, et al. Guía de práctica clínica sobre el manejo del estreñimiento crónico en el paciente adulto. Parte 2: Diagnóstico y tratamiento. Gastroenterol Hepatol 2016; en prensa. [ Links ]
155. Rao SSC, Bharuca AE, Chiarioni G, et al. Anorectal Disorders. Gastroenterol 2016; 150: 1430-1442. [ Links ]
![]() Correspondence:
Correspondence:
Javier Júdez.
Knowledge Management Department Director.
Sociedad Española de Patología Digestiva
(Spanish Society of Digestive Diseases).
C/ Sancho Dávila, 6. 28028
Madrid, Spain
e-mail: jjudez@sepd.es
Received: 18-04-2016
Accepted: 19-04-2016











 texto en
texto en