Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.9 Madrid sep. 2016
https://dx.doi.org/10.17235/reed.2015.3929/2015
CASE REPORTS
Rare association of celiac disease with myasthenia gravis in a patient with other immune disorders: a Case Report
Marcela de Almeida-Menezes1, Vírginia Lúcia Ribeiro-Cabral2, Sônia S. Lorena2, Anamarli Nucci3, Priscila Andrade-Santana2 and Cecília Queiroz Silva2
Departments of 1Internal Medicine,2Internal Medicine-Gastroenterology and
3Neurology. State University of Campinas-Unicamp. São Paulo, Brazil
ABSTRACT
Background: Celiac disease is described in association with several autoimmune diseases, but rarely with myasthenia gravis.
Case Report: We describe the case of a 31-year-old white woman with celiac disease who presented manifestations related to a hyperactive immune system, including macroamylasemia, false-positive anti-HCV, positive antinuclear antibody, and Raynaud's phenomenon. The Introduction of a gluten-free diet (GFD) resolved these features, but myasthenia gravis (MG) symptoms unexpectedly occurred on that occasion.
Discussion: The role of a GFD in the course of autoimmune diseases has been studied and improvement has been reported in many diseases. However, there is no consensus in the literature regarding the course of neurological disorders associated with celiac disease. In the present case, a GFD did not prevent the appearance of symptoms related to myasthenia gravis. There are few reports on the association of celiac disease with myasthenia gravis and therefore little is known about the course and time of onset of myasthenia in celiac patients. The present case increases the knowledge about this unusual autoimmune neurological disease associated with celiac disease.
Key words: Celiac disease. Miasthenya gravis. Gluten-free diet.
Introduction
Celiac disease (CD) is described in association with several autoimmune diseases (1) and neurological disorders, and up to 10% of CD patients develop neurological complications (2). Myasthenia gravis (MG) is an autoimmune neuromuscular disorder that is rarely described in patients with CD (3). We report the case of a patient with CD who presented manifestations related to a hyperactive immune system, including macroamylasemia, false-positive anti-HCV, positive antinuclear antibody, Raynaud's phenomenon, and MG. The administration of a gluten-free diet (GFD) caused rapid resolution of diarrhea and regression of immune events, but symptoms of MG unexpectedly occurred on that occasion.
Case Report
A 31-year-old woman was seen at our gastroenterology service with a 9-year history of intermittent diarrhea, associated with abdominal pain and weight loss of 12 kg. Previous laboratory investigation at another service had shown persistent hyperamylasemia and anemia. The patient had a history of an episode of Raynaud's phenomenon associated with intensification of diarrhea one year earlier and reported worsening of diarrhea in the last 4 months, as well as hypotension, hyporexia and muscle weakness.
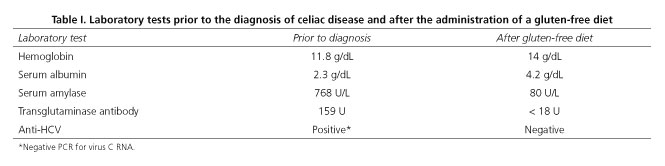
Upper gastrointestinal endoscopy showed erosive and nodular bulboduodenitis and the duodenal biopsy findings included moderate to severe chronic erosive duodenitis and villus atrophy. Anti-tissue transglutaminase IgA autoantibodies were positive. The patient exhibited no symptoms consistent with acute pancreatitis, and other non-pancreatic causes of hyperamylasemia in CD patients were therefore investigated. Measurement of serum and urine amylase levels showed low amylase clearance, consistent with macroamylasemia. The Results of laboratory tests included positive antinuclear antibody (1/160, coarse reticular speckled pattern) and positive anti-HCV. PCR for virus C RNA and anti-dsDNA, anti-RNP and anti-SM were negative. Nailfold capillaroscopy was normal.
After the diagnosis of CD, a GFD was introduced and the patient exhibited resolution of diarrhea and weight loss. Anti-transglutaminase antibodies and anti-HCV subsequently became negative and amylasemia was normalized (Table I).
During follow-up, about one year after the Introduction of the GFD, the patient developed asthenia, diplopia, headache, and worsening of weakness in the absence of associated gastrointestinal symptoms. Neurological examination showed rapid muscle fatigue on repetitive exercise. Limb muscle strength was reduced after prolonged physical exercise and palpebral ptosis on sustained upward gaze was observed. Diplopia occurred after eye lateralization for 20 seconds. Reflexes and sensory testing were normal. Electroneuromyography revealed diffuse myopathic changes consistent with MG. Acetylcholine receptor antibodies were positive (4.71 nmol/L, reference: positive > 0.5 nmol/L). Computed tomography showed no evidence of thymoma. The diagnosis of MG was made and treatment with prednisone and azathioprine was initiated.
Discussion
Celiac disease is an autoimmune T cell-mediated disorder triggered by the ingestion of gluten in genetically predisposed individuals. More than 99% of patients express HLA DQ2 or DQ8 (2). The prevalence of autoimmune diseases is higher in patients with CD when compared to the healthy control population. The cumulative risk of autoimmune disease in patients with CD is 8% at age 15 and 16% at age 30. Exposure to gluten seems to contribute to the emergence of autoimmunity and patients who adhere to a GFD have a lower risk of developing autoimmune diseases (1). The observation of normalization of immune events in the present case after Introduction of the GFD supports this hypothesis. However, even within this context, the patient developed symptoms related to MG.
Macroamylasemia is a benign condition caused by circulating macroamylase complexes of pancreatic or salivary amylase bound to plasma proteins, which cannot be cleared by the renal glomeruli. In most cases, the macromolecular amylase represents a complex of normal amylase and either immunoglobulin A or G, and may be a specific antigen-antibody complex (4). There are reports of CD associated with macroamylasemia, with disappearance of this macroenzyme after the Introduction of a GFD (5,6), as observed in the present patient. The understanding of this event could prevent patients from being submitted to extensive investigation of pancreatic diseases. The frequency of macroamylasemia has been shown to be higher among patients newly diagnosed with CD than in those receiving a GFD and in these two populations compared to controls without CD (7), a fact demonstrating the role of gluten in the occurrence of this disorder. With respect to the association of CD with Raynaud's phenomenon, few data are available in literature. The rationale for this association could be the common autoimmune pathogenetic background of both disorders. Moreover, the strong immunoinflammatory activation observed in active CD that triggers the chronic release of several cytokines endowed with vasoactive properties (interferon-gamma and interleukin -2, -4, -10) might affect arterial regulation in predisposed patients (8). There are reports indicating a reduction in Raynaud's phenomenon after the Introduction of a GFD and vitamin reposition (9,10).
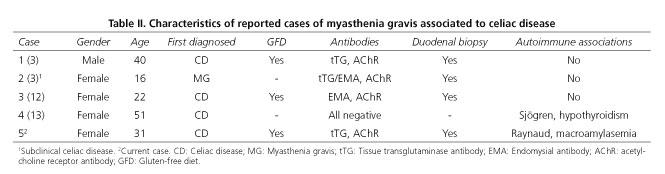
Little is known about the association of CD with myasthenia gravis. Both conditions are T cell-mediated autoimmune diseases in which tissue transglutaminase and nicotinic acetylcholine receptors are the target antigens of the immune attack, respectively (2). The association between CD and myasthenia gravis could be coincidental, but available evidence suggests that these two distinct immune-mediated disorders occur together more frequently than is currently appreciated. Similar human leukocyte antigen types (HLA -DR3, -DQ2,-DQ8) seem to predispose to both MG and CD (3,11). Some serological surveys have investigated the concurrence of antibodies present in the two diseases, with no expressive Results (2,3). We found three studies in the literature reporting the association of these two diseases in which the diagnosis of CD preceded symptoms of MG (3,12,13), and one case in which subclinical CD was diagnosed after serological screening of tTG and EMA in patients with myasthenia gravis, confirmed by duodenal biopsy (3) (Table II).
There are reports of the occurrence of MG even after the Introduction of a GFD in patients with CD (3,12). The role of a GFD in the course of autoimmune diseases has been studied, but there is no consensus in the literature regarding the course of neurological disorders associated with CD. These disorders sometimes become symptomatic after long periods of exclusion of gluten from the diet (14), as observed in our patient. Muscle weakness in patients with malabsorption might be related to malnutrition or electrolyte disorders. In the present case, aggravation of this symptom was observed after the restrictive diet and in the absence of intestinal symptoms. The diagnosis of accompanying MG may be delayed if weakness is ascribed to CD. The presence of motor weakness and diplopia in treated CD may be a clue to occult myasthenia gravis (11).
References
1. Freeman HJ, Chopra A, Clandinin MT, et al. Recent advances in celiac disease. World J Gastroenterol 2011;17(18):2259-72. DOI: 10.3748/wjg.v17.i18.2259. [ Links ]
2. Briani C, Doria A, Ruggero S, et al. Antibodies to muscle and ganglionic acetylcholine receptors (AChR) in celiac disease. Autoimmunity 2008;41(1):100-4. DOI: 10.1080/08916930701619987. [ Links ]
3. Freeman HJ, Gillett HR, Gillett PM, et al. Adult celiac disease with acetylcholine receptor antibody positive myasthenia gravis. World J Gastroenterol 2009;15(38):4741-44. DOI: 10.3748/wjg.15.4741. [ Links ]
4. Barera G, Bazzigaluppi E, Viscardi M, et al. Macroamylasemia attributable to gluten-related amylase autoantibodies: A Case Report. Pediatrics 2001;107(6):E93. DOI: 10.1542/peds.107.6.e93. [ Links ]
5. Depsames R, Fireman Z, Niv E, et al. Macroamylasemia as the first manifestation of celiac disease. Case Rep Gastroenterol 2008;2(2):196-198. DOI: 10.1159/000132771. [ Links ]
6. Liu Z1, Wang J, Qian J, et al. Hyperamylasemia, reactive plasmacytosis, and immune abnormalities in a patient with celiac disease. Dig Dis Sci 2007;52(6):1444-7. Epub 2007 Apr 19. DOI: 10.1007/s10620-006-9268-0. [ Links ]
7. Rabsztyn A, Green PHR, Berti I, et al. Macroamylasemia in patients with celiac disease. Am J Gastroenterol 2001;96:1096-1100. DOI: 10.1111/j.1572-0241.2001.03746.x. [ Links ]
8. Gabrielli M, Candelli M, Santarelli L, et al. Raynaud's phenomenon and celiac disease. Am J Gastroenterol 2003;98(11):2578-9. DOI: 10.1111/j.1572-0241.2003.08680.x. [ Links ]
9. Hozyasz K, Czerwi?ska B. Atypical celiac disease in an adolescent girl - Case Report. Pol Merkur Lekarski 2004;17(101):491-3. [ Links ]
10. Thonhofer R, Trummer M, Siegel C. Capillaroscopy shows an active pattern of scleroderma in coeliac disease. Scand J Rheumatol 2010;39(5):438-9. DOI: 10.3109/03009742.2010.489230. [ Links ]
11. Kocsis D, Csaplar M, Jocsak E, et al. Celiac disease association with other autoimmune disorders: Three Case Reports. Case Reports in Internal Medicine 2015;2:23-9. [ Links ]
12. Csaplár M1, Juhász M, Muzes G, et al. Association of coeliac disease and myasthenia gravis. Orv Hetil 2006;147(18):841-4. [ Links ]
13. Innico G, Frassetti N, Coppola B, et al. Autoimmune polyglandular syndrome in a woman of 51 years. Eur Rev Med Pharmacol Sci 2014; 18(12):1717-9. [ Links ]
14. Luostarinen L, Himanen S-L, Luostarinen M, et al. Neuromuscular and sensory disturbances in patients with well treated coeliac disease. J Neurol Neurosurg Psychiatry 2003;74:490-4. DOI: 10.1136/jnnp.74.4.490. [ Links ]
![]() Correspondence:
Correspondence:
Marcela de Almeida-Menezes.
Department of Internal Medicine.
State University of Campinas-Unicamp.
Cidade Universitária Zeferino Vaz. Barão Geraldo, Campinas.
13083-970 São Paulo, Brazil
e-mail: marcelaamenezes@gmail.com
Received: 16/07/2015
Accepted: 16/07/2015