Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.108 no.10 Madrid oct. 2016
https://dx.doi.org/10.17235/reed.2016.4229/2016
ORIGINAL PAPERS
Epidemiology of Barrett's esophagus and esophageal adenocarcinoma in Spain. A unicentric study
Epidemiología del esófago de Barrett y adenocarcinoma esofágico en España. Estudio unicéntrico
Quetzalihuitl Arroyo-Martínez1, Manuel Rodríguez-Téllez1, Antonio García-Escudero2, Javier Brugal-Medina2, Ricardo González-Cámpora2 and Ángel Caunedo-Álvarez1
1Gastroenterology Intercenter Unit. Hospitales Universitarios Virgen Macarena-Virgen del Rocío. Seville, Spain
2Anatomical Pathology Unit. Hospital Universitario Virgen Macarena. Seville, Spain
ABSTRACT
Background: Barrett's esophagus (BE) is an acquired disease defined by the presence of intestinal metaplasia with goblet cells in the distal esophagus. The prevalence of BE has increased dramatically over the last years.
Aims: The primary aims of the study were to analyze the characteristics of BE and esophageal adenocarcinoma (EAC) in a Spanish health district during a follow-up period.
Methodology: Sociodemographic factors, alcohol consumption and cigarette smoking were analyzed. We also studied the histological behavior and cause of death in each group.
Results: In the present study 430 patients were included, 338 with BE and 92 with EAC. Incidence rates have risen from 2.25 and 1.25 per 100,000 inhabitants in 1996 to 6.5 and 4.75 per 100,000 in 2011, respectively. In the EAC group, male gender, age and alcohol consumption were higher in comparison to the BE group, and the overall survival was 23 months. In the BE group, the main causes of death were non-esophageal cancer and cardiovascular disease.
Conclusions: The incidence and prevalence rates of AEC and BE have risen over the past years. Risk factors for these conditions were male gender, age and alcohol consumption. Long BE (> 3 cm) is involved in dysplasia progression. AEC diagnosis mainly occurs after neoplasia is detected and, in a few cases, due to a previous BE. Cardiovascular diseases and non-esophageal cancers have been found to be the main cause of death in BE patients.
Key words: Esophageal adenocarcinoma. Barrett's esophagus. Metaplasia. Dysplasia.
RESUMEN
Introducción: el esófago de Barrett (EB) es una enfermedad adquirida definida por la presencia de metaplasia intestinal en el esófago distal. Su prevalencia se ha incrementado de forma alarmante en los últimos años.
Objetivos: los objetivos primarios del presente trabajo fueron analizar el comportamiento del EB y del adenocarcinoma esofágico (ACE) en un área sanitaria española durante el seguimiento del periodo del estudio.
Métodos: se analizaron características sociodemográficas y el consumo de alcohol y tabaco. También se valoró el comportamiento histológico así como las causas de defunción en cada uno de los grupos.
Resultados: se incluyeron 430 pacientes, 338 con EB y 92 con ACE. La tasa de incidencia pasó de 2,25 y 1,25 por 100.000 habitantes en 1996 a 6,5 y 4,75 en 2011, en EB y ACE, respectivamente. Hubo más varones, mayor edad e ingesta etílica en el grupo adenocarcinoma respecto al grupo de Barrett. La supervivencia del ACE fue de 23 meses. Las principales causas de muerte en los pacientes con Barrett fueron el cáncer no esofágico y la enfermedad cardiovascular.
Conclusiones: existe una mayor incidencia y prevalencia tanto del EB como del ACE en los últimos años. Como factores de riesgo encontramos el sexo masculino, mayor edad y consumo de alcohol. El EB largo (> 3 cm) está implicado en la progresión de la displasia. El diagnóstico de ACE se hace, la mayor parte de las veces, con el debut de la enfermedad neoplásica y, en el menor de los casos, sobre un EB previo. La enfermedad cardiovascular y neoplásica no esofágica han sido las principales causas de mortalidad en los pacientes con EB.
Palabras clave: Adenocarcinoma esofágico. Esófago de Barrett. Metaplasia. Displasia.
Introduction
Barrett's esophagus (BE) is an acquired disease defined by the presence of intestinal metaplasia changes in the distal esophagus in which the normal squamous epithelial cells are replaced by columnar epithelium, mainly as a result of gastroesophageal reflux disease (GERD) (1,2). The actual prevalence of GERD among the general population remains unknown. Recent studies performed in Western countries have shown that 20-25% of the adult population experience GERD symptoms at least once a week (3,4). In Europe, 10% of the population report GERD symptoms at least once a week and 20% experience them occasionally, with an approximate annual rate of 4% (5-7). Regarding BE, the overall estimated incidence in the general population is 1 to 2% (8-10). According to epidemiological studies performed in Endoscopy units, when endoscopic examinations are carried out in the absence of GERD symptoms, BE is observed in 0.05-2% of cases. However, when preceded by GERD symptoms, it may be seen in up to 12% of cases. In the last years, prevalence of BE in Southern Europe has alarmingly increased from 6.51/100,000 inhabitants to 76.04/100,000 inhabitants (1985-2001, respectively) (11-13). In other countries, a significant increase has also been observed in the general population, ranging from 0.08-2% in the period 1987-1996 to 4% in 2004 (14-17). The main concern of BE lies in its neoplastic potential, as the overall risk of progression to EAC is 0.5-1% per year (18). Nevertheless, and despite all the available diagnostic tests, up to 40% of EAC patients do not refer to previous GERD symptoms, thus most of the cases are diagnosed in an advanced phase (19).
So far, the relationship between GERD and the development of BE and EAC remains unexplained, although some factors are thought to increase this risk (genetic factors, age, male gender, obesity, alcohol consumption and cigarette smoking).
The main aim of this study was to analyze the behavior of BE and EAC in a Spanish health district during a specific follow-up period. Our secondary aims were to study those risk factors associated with BE and the histological behavior of BE during that period, evaluating survival and cause of death in these patients.
Material and methods
Authorization from the Ethics Committee was obtained in order to include patients in this study. Patients with BE and/or EAC histologically confirmed results between 1996 and 2011 were included. Those with endoscopic diagnosis unconfirmed by histology, esophageal cancer of different histological entity or gastric carcinoma affecting the esophagus were excluded.
Dysplasia was classified according to the criteria suggested by the World Health Organization (WHO) (20), based on those proposed by the Inflammatory Bowel Disease-Dysplasia Morphology Study Group (21). All samples were first studied by a qualified pathologist in order to define the type of BE and to describe changes suggestive of dysplasia. Confirmation of EAC or the grade of dysplasia was given by a second pathologist, following the established protocol.
The follow-up period was calculated from the date of BE diagnosis to the last endoscopy with an esophageal biopsy. The survival period was defined as the time period between the first diagnosis and the last check-up or death. Patients that fulfilled the inclusion criteria were initially divided in two groups: a) BE; and b) EAC. We assessed demographic features such as gender, age, cigarette smoking (positive when smoking index was > 10), alcohol consumption (positive when > 15 units/week in men and > 12 units/week in women), follow-up period (months) and survival (months) in each group. Subsequently, they were divided into four subgroups according to the reported histological behavior, ratified by AP results during the study period. Group 1: "BE without dysplasia" (BEWoD); group 2: "BE with stable dysplasia" (BESD), defined by the presence of dysplasia without progression and/or regression during the follow-up period; group 3: "BE with dysplasia progression" (BEDP), those with varying histopathological characteristics during the study period (following the sequence of metaplasia - low-grade dysplasia/LGD - high-grade dysplasia /HGD - EAC); and group 4: "EAC", without previous BE diagnosis.
Sociodemographic aspects were also studied (gender, age, cigarette smoking and alcohol consumption), as well as BE length measured by endoscopy, survival rate and cause of death in each group. Regarding BE length, as most of the results were obtained prior to the establishment of the Prague criteria (C, circumferential extent, and M, maximum extent) (22,23), BE was measured in centimeters, counting from the esophageal gastric junction (EGJ) to the proximal section of the squamocolumnar junction hence distinguishing short BE (< 3 cm) from long BE (> 3 cm). Once these data were obtained for each group, a multivariate comparison analysis was carried out, with the purpose of determining possible risk factors with an influence on the histopathological behavior of BE and EAC.
Statistical analyses
Qualitative and quantitative variables were studied. Quantitative variables were expressed as means and standard deviation (SD) and qualitative variables were presented as the total number of events and percentages. Quantitative independent variables were assessed using the t-Student test and the non-parametric Mann-Whitney U test, whilst non-dichotomous variables were studied using the analysis of variance test (ANOVA). Qualitative variables were assessed using Pearson's Chi-squared test (χ²). Survival was analyzed using Kaplan Meier curves. All statistical tests were carried out using the SPSS software (version 19, IBM®, Chicago, IL) and a p value < 0.05 was considered as statistically significant.
Results
Incidence rates of BE and EAC
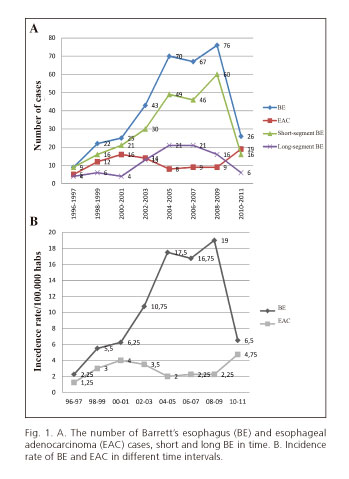
According to the study periods and taking into account our reference population (400,000 inhabitants older than 15 years old), the highest incidence rate (IR) was seen between January 2008 - December 2009 (19/100,000 people/year) and January 2010 - December 2011 (4.75/100,000 people/year) for BE and EAC, respectively, predominantly with a short BE (250 cases, 74%) (Fig. 1). IR was calculated for each age group (Fig. 2A), the highest rates were found in patients of 50-59 years of age (20/100,000 people/year) and 70-79 years of age (6.5/100,000 people/year) for BE and EAC, respectively. Histological behavior was also analyzed by age groups (Fig. 2B), with most cases corresponding to BE without dysplasia, especially between 50-59 years of age (IR 16.5/100,000 people/year).
Sociodemographic characteristics per group
BE vs. EAC group
Sociodemographic characteristics of each group are shown in table I. We studied 430 patients, most of them with short BE (74% of 79% of the cases), whereas 92 patients (21%) had EAC without a previous BE diagnosis. In both groups, most patients were men (75% and 88% in the BE and EAC group, respectively), which was statistically significant when compared to the percentage of women (p < 0.05). The mean age was 53 and 67 years old in the BE and EAC groups, respectively (p < 0.05). Likewise, prior alcohol consumption was reported in both groups (52% and 81%, respectively) as well as cigarette smoking (63% and 76%, respectively), with a statistically significant association in the case of alcohol consumption (p < 0.05 [CI 95% 1.4-9.5]) but not smoking (p = 0.16 [CI 95% 0.7-4.1]).
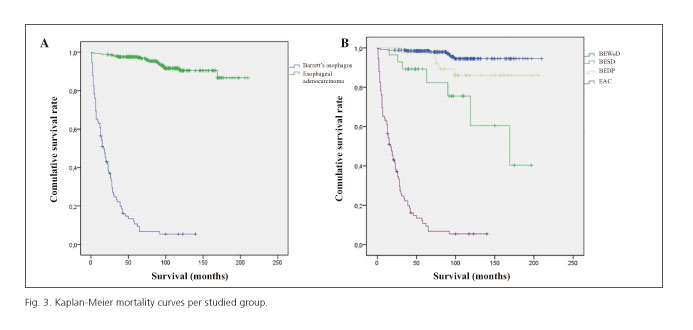
The mean follow-up time was 30 months in BE patients (0-175) and 23.9 months in EAC patients (1-140), which was statistically significant (p = 0.01). BE patients survival was statistically higher than EAC (92 vs. 24 months, p < 0.05) and overall mortality was 6.2% vs. 89%, respectively (p < 0.05) (Fig. 3A).
Subgroup analyses
Most patients (62.7%) had BEWoD, and males were more common in all groups. The youngest population was found in the BEWoD group (χ = 53 years old) whereas the oldest were seen in the EAC group (χ = 66.6 years). Body mass index (BMI) was calculated in 23 patients from the BE group (7% of the studied population) and 11 from the EAC group (12%), showing a higher BMI in patients with BE with dysplasia (28.09 kg/m2) in comparison to the BEWoD and the EAC groups (25.11 kg/m2 and 23.6 kg/m2, respectively).
We observed a higher proportion of patients with short BE in the BEWoD and BESD groups when compared to the BEDP, which was statistically significant (p < 0.01 and p = 0.014 [CI 95% 2.2-8.6] and 0.27 [CI 0.09-0.7], respectively). Also, alcohol consumption was higher in the EAC group than in the BEWoD and BESD (p = 0.03 and p = 0.044, respectively).
The highest survival period was observed in BEDP patients (115.3 months), and was statistically significant when compared to the other groups (p < 0.01) (Table II and Fig. 3B). On the contrary, mortality was significantly higher in the EAC group (p < 0.001).
During follow-up, 338 patients with BE were studied (270 w/o dysplasia, 28 with stable dysplasia and 40 with histological changes). Eight patients progressed to EAC (2.4%), two of which (25%) died due to complications derived from their disease. One of them had HGD in his first endoscopy and passed away 31 months later; the other one exhibited histological changes, from intestinal metaplasia to EAC during follow-up, and passed away 60 months after initial diagnosis.
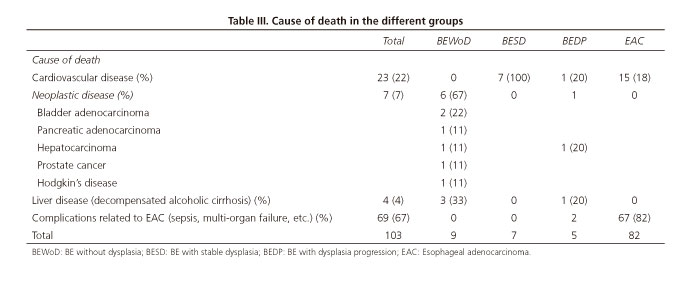
The main causes of death in BE patients were those related to cardiovascular diseases and non-esophageal cancer (Table III).
Discussion
Nowadays, neoplastic diseases continue to be a frequent cause of morbidity and mortality in developed countries, only preceded by cardiovascular diseases as a cause of death in the adult population. In the last years, different studies have reported a decrease in most of the prevalent types of cancer, yet other types such as pancreatic, liver, thyroid, kidney and esophageal adenocarcinoma have increased, with up to a six-fold increase in the latter (24-26).
In the meta-analysis published by Yousef et al. (27), the incidence of AEC among patients with BE was 5.3 per 1,000 persons/year. However, Gilbert et al. (28) described that BE could affect from 1.6% to 6.8% of the general population. In our series, BE and AEC incidence rates have increased from 2.25 and 1.25 per 100,000 inhabitants in 1996 to 6.5 and 4.75 per 100,000 in 2011, respectively. This represents an increase of more than 350% during the last years.
Most EAC cases were newly diagnosed, without a previous BE diagnosis. In the series published by Corley (29), the EAC incidence among BE cases was only 5% whilst in our series it represented 2.4% of all cases. Analyses of the studied risk factors are also included.
Gender
In this study, we replicated the observations made by most authors, that the incidence in men is much higher than in women in both BE and EAC cases. Yousef has calculated an incidence rate of EAC in previous BE of 10.2/1,000 persons/year in men and 4.5/1,000 persons/year in women. Kubo et al. (30) carried out a study that included patients from 1973 to 1998, finding that the risk of developing EAC was 8 times higher in men than in women; furthermore, Cook et al. (31) described that men were not only more susceptible to the development of BE but, once established, the risk of progression to cancer was also higher. In the present study, 75% of the patients with BE were men, whilst this proportion rose up to 88% in the EAC group.
Age
Determining how much risk age represents in the development of asymptomatic diseases, such as BE, is a difficult task. Nearly all the results described by Corley et al. (32) were obtained in an indirect manner; mostly using different databases form Endoscopy units. The incidence of BE adjusted to the volume of endoscopies performed was 7/100,000 persons/year in the age range from 21-30 years (the lowest one registered), slowly increasing until its highest value in the 61-70 years age range (31/100,000 person/year). In the present study, the highest age range was almost a decade younger, showing an incidence rate of 20/100,000 persons/year, in the 50-59 years age range. On the other hand, the highest incidence rate of EAC is observed two decades later, 6.5/100,000 persons/year in the range of 70-79 years of age.
There are several factors that could explain the higher incidence during the 5th-6th decade of life, such as the accumulative effect of the damage caused by gastric and bile acids/bases on the esophageal mucosa caused by GERD which may eventually lead to metaplastic changes as a mechanism of defense.
Another interesting observation is the study of histological behavior according to the different age ranges. Hence, as the population grows older, the number of cases with progressive histological changes also increases, reaching its highest incidence in the range of 60-69 years of age; it then remains stable until the next decade. This ascending pattern of dysplasia is quite similar to that observed for EAC. However, we must take into account that ageing is also accompanied by other factors, such as the carcinogenic factor per se of cellular ageing or the acquisition of different habits which may be even more pernicious than age itself. If we take as an example the National Survey of Health in Spain, published by the Ministry of Health, Equality and Social Policies (33), the age range with a higher cigarette smoking rate is from 50 to 60 years of age (34% of men and 30% of women, approximately). This survey also highlights that obesity (another factor involved in the appearance of BE) has increased from 7.4% to 17% in the last 25 years, especially in men (around 18% of adult male population have a BMI > 30 kg/m2). This percentage increases in a direct proportion with age, until reaching 74 years, when it starts to decrease.
Obesity
In this study, the BMI of patients with BE and dysplasia was higher in comparison to those with BEWoD and EAC (28 kg/m2 vs. 25.11 kg/m2 and 23.6 kg/m2, respectively). Regarding the fact that the sample size was small, our results are consistent with those obtained by Cook et al. (34), in which only 10 out of 295 studies fulfilled the inclusion criteria. The odds ratio (OR) for adiposity was 0.99 per kg/m2, concluding that the traditional risk attributable to adiposity is probably lower than expected. Cook's observations have been supported by another study conducted by Kubo et al. (35). This paper included 14 studies in which 2,488 cases of EAC and 2,509 cases of GEJ cancer were analyzed. Patients with a BMI > 25 kg/m2 had a higher risk of developing EAC (OR 2.2 in men, 2.0 in women) as well as GEJ cancer (OR 1.5). Based on Vaughan's (36) findings, histological, genetic and cytometric variations are observed in patients with abdominal adiposity, but not in those with elevated BMI, leading to the conclusion that the attributable risk of adiposity was due to central obesity (hip-waist index < 1), mostly observed in men.
There is another research line that assesses the role of insulin resistance in the obese population, which has already been proved to be related to other types of cancer (breast, prostate, lung, colon and rectum), as stated by Yu (37).
Alcohol consumption and cigarette smoking
An observation of this study is that most patients with BE are alcohol abstainers, which was statistically significant in comparison to those with EAC. According to histological data, there were more patients that consumed alcohol in the EAC group than in the BEWoD or BESD group. These results are in accordance with those obtained by Veugelers et al. (38), in which alcohol is a risk factor associated with progression of BE. This, however, differs from more recent studies conducted by Thrift et al. (39). In the latter, the author has reviewed the database of the BEACON Consortium, which includes more than 3,000 patients. After comparison with control subjects, it was found that low levels of daily alcohol consumption (3-5 alcohol beverages with low alcohol content) could act as a protective factor in BE whilst higher consumption was apparently not related to the presence/absence of BE or EAC.
With regards to cigarette smoking, there is clear evidence of its carcinogenic potential associated with EAC. Thus, the study conducted by Cook et al. (40) is well worth mentioning, in which a direct association is reported between cigarette smoking and the incidence of BE, with an ascending slope that reaches a plateau in 20 packs/year.
Length of Barrett's esophagus
Regarding the length of BE (short vs. long) and its relationship with histological behavior, our study shows that short BE (< 3 cm) seems to display a more benign behavior as most of them do not develop dysplasia or, when they do, it remains stable. Identifying this factor as a risk factor in the progression of dysplasia and EAC is not an easy task, as all published series are quite heterogeneous and have shown conflicting results. In this regard, the study by Anaparthy et al. (41) stands out. Among its results, 1,175 patients were studied, finding that in those with BE > 3cm the risk of developing HGD or EAC was increased by 28% for each additional centimeter. This observation was replicated in the present study, as the length of BE was < 3 cm in 78% of BEWoD cases whilst in 55% of the cases in which histological changes were more aggressive and sustained degeneration/progression to dysplasia was observed, BE length was > 3 cm. In a more recent study, Pohl et al. (42) question the effectiveness of testing programs, especially in short and ultra-short BE. In this review of 1,017 patients with EAC stage T1 they prove that, in order to detect EAC over a previous BE diagnosis, annual endoscopy should be performed in 450 patients with long BE, in 3,440 patients with short BE and in the majority of 12,364 patients with ultra-short BE.
Overall survival in the EAC group was lower in comparison to the other groups, with a mean of 23 months. However, it was quite unexpected that survival was longer in those patients with progression of dysplasia, and the highest mortality rate, except for the EAC group, was observed in the BESD group. This result, although contradictory at first, is explained after analyzing the population in each group as well as the different causes of death. The number of deaths in the BESD group was higher (7 patients, 25% of the sample) and the BMI values were also higher in this group. These factors seem to be directly related to the causes of death in this group, as death did not occur as a consequence of esophageal disease but rather due to cardiovascular pathologies. The percentage of deaths in patients without dysplasia remained relatively low (3.3%). These results support the observations shown by other study groups (43-45) according to which patients with BE are quite similar to the general population as cardiovascular risk factors are the main determinants of survival.
EAC appeared in 8 out of 40 cases in which dysplasia progressed, all of them in relatively short periods of time (15-60 months). In five of these cases, BE progressed straight from intestinal metaplasia to EAC, without any evidence of dysplasia in screening biopsies. Thus, dysplasia was observed in only three of the cases that subsequently developed EAC. This finding can be explained by several reasons, maybe the most important one linked to insufficient biopsies, inadequate histopathological interpretation, or a combination of both.
In summary, in the present study both incidence rate and prevalence of Barrett's esophagus and esophageal adenocarcinoma have increased in the last years. In both entities, male gender has been found to be a risk factor. Age and alcohol consumption are related to the development of EAC. Long BE (> 3 cm) has been the only factor related to the progression of BE. In most cases, EAC is diagnosed with the onset of neoplastic disease and in a minority of cases due to a previous BE. Cardiovascular diseases and non-esophageal neoplasia were found to be the main determinants in BE patients.
References
1. Hamilton Sr. Esophagitis. In: Phatology of the Gastrointestinal Tract. Second edition. Siu-Chun Ming, Harvey Goldman, Eds. Williams and Wilkins. 1998;20:433-74. [ Links ]
2. Nason KS, Wichienkuer PP, Awais O, et al. Gastroesophageal reflux disease symptom severity, proton pump inhibitor use, and esophageal carcinogenesis. Arch Surg 2011;146: 851-8. DOI: 10.1001/archsurg.2011.174. [ Links ]
3. Moore M, Afaneh C, Benhuri D, et al. Gastroesophageal reflux disease: A review of surgical decision making. World J Gastrointest Surg 2016;8:77-83. DOI: 10.4240/wjgs.v8.i1.77. [ Links ]
4. Ciriza-de-los-Ríos C. Barrett's esophagus: A review. Rev Esp Enferm Dig 2010;102:257-69. DOI: 10.4321/S1130-01082010000400006. [ Links ]
5. King A, MacDonald C, Orn C. Understanding gastro-oesophageal reflux disease: A patient-cluster analysis. Int J Clin Pract 2008;62:1838-43. DOI: 10.1111/j.1742-1241.2008.01929.x. [ Links ]
6. Díaz-Rubio M, Moreno-Elola-Olaso C, Rey E, et al. Symptoms of gastro-oesophageal reflux: Prevalence, severity, duration and associated factors in a Spanish population. Aliment Pharmacol Ther 2004;191:95-105. DOI: 10.1046/j.1365-2036.2003.01769.x. [ Links ]
7. Rey E, Álvarez-Sánchez A, Rodríguez-Artalejo F, et al. Onset and disappearance rates of gastroesophageal reflux symptoms in the Spanish population, and their impact on quality of life. Rev Esp Enferm Dig 2009;101:477-82. DOI: 10.4321/S1130-01082009000700005. [ Links ]
8. Hamilton SR, Smith RR, Cameron JL. Prevalence and characteristics of Barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Hum Pathol 1988;19:942-8. DOI: 10.1016/S0046-8177(88)80010-8. [ Links ]
9. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: An endoscopic study. Gastroenterology 2005;129:1825-31. DOI: 10.1053/j.gastro.2005.08.053. [ Links ]
10. Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med 2014;371: 836-45. DOI: 10.1056/NEJMra1314704. [ Links ]
11. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology 2002;123:461-7. DOI: 10.1053/gast.2002.34748. [ Links ]
12. Alcedo J, Ferrández A, Arenas J, et al. Trends in Barrett's esophagus diagnosis in Southern Europe: Implications for surveillance. Dis Esophagus 2009;22:239-48. DOI: 10.1111/j.1442-2050.2008.00908.x. [ Links ]
13. Sánchez Robles C, Santalla Peciña F, Retamero Orta MD. Barrett esophagus. An epidemiological study in an area of Spain. Rev Esp Enferm Dig 1995;87:353-5. [ Links ]
14. Bytzer P, Christensen PB, Damkier P, et al. Adenocarcinoma of the esophagus and Barrett's esophagus: A population-based study. Am Gastroenterol 1999;94:86-91. DOI: 10.1111/j.1572-0241.1999.00776.x. [ Links ]
15. Prateek S. The worldwide prevalence of Barrett's esophagus. World Gastroenterology News 2001;1:22-5. [ Links ]
16. Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: A systematic review. Gastroenterology 2002;122:26-33. DOI: 10.1053/gast.2002.30297. [ Links ]
17. Ford AC, Forman D, Reynolds PD, et al. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett's esophagus. Am J Epidemiol 2005;162:454-60. DOI: 10.1093/aje/kwi218. [ Links ]
18. Wang KK, Sampliner RE, Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol 2008;103:788-97. DOI: 10.1111/j.1572-0241.2008.01835.x. [ Links ]
19. Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31. DOI: 10.1056/NEJM199903183401101. [ Links ]
20. Flejou J F. WHO Classification of digestive tumors: The fourth edition. Ann Pathol 2011;31:S27-31. [ Links ]
21. Riddell RH, Goldman H, Ransohoff DE, et al. Dysplasia in inflammatory bowel disease: Standardized classification with provisional clinical information. Hum Pathol 1983;14:931-68. DOI: 10.1016/S0046-8177(83)80175-0. [ Links ]
22. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: The Prague C & M criteria. Gastroenterology 2006;13:1392-9. DOI: 10.1053/j.gastro.2006.08.032. [ Links ]
23. Vahabzadeh B, Seetharam AB, Cook MB, et al. Validation of the Prague C & M criteria for the endoscopic grading of Barrett's esophagus by gastroenterology trainees: A multicenter study. Gastrointest Endosc 2012;75:236-41. DOI: 10.1016/j.gie.2011.09.017. [ Links ]
24. Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-28. DOI: 10.3322/caac.20141. [ Links ]
25. Dubecz A, Solymosi N, Stadlhuber RJ, et al. Does the incidence of adenocarcinoma of the esophagus and gastric cardia continue to rise in the twenty-first century? - A SEER database analysis. J Gastrointest Surg 2014;18:124-9. DOI: 10.1007/s11605-013-2345-8. [ Links ]
26. Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett's esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am 2015;44:203-31. DOI: 10.1016/j.gtc.2015.02.001. [ Links ]
27. Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: A systematic review and meta-analysis. Am J Epidemiol 2008;168:237-49. DOI: 10.1093/aje/kwn121. [ Links ]
28. Gilbert EW, Luna RA, Harrison VL, et al. Barrett's esophagus: A review of the literature. J Gastrointest Surg 2011;15:708-18. DOI: 10.1007/s11605-011-1485-y. [ Links ]
29. Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett's adenocarcinomas: A population-based study. Gastroenterology 2002;122:633-40. DOI: 10.1053/gast.2002.31879. [ Links ]
30. Kubo A, Corley DA. Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer 2002;95:2096-102. DOI: 10.1002/cncr.10940. [ Links ]
31. Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol 2005;162:1050-61. DOI: 10.1093/aje/kwi325. [ Links ]
32. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett's oesophagus diagnosis: A large community-based study, 1994-2006. Gut 2009;58:182-8. DOI: 10.1136/gut.2008.163360. [ Links ]
33. Instituto Nacional de Estadística. Ministerio de Sanidad, Servicios Sociales e Igualdad. Encuesta Nacional de Salud 2011-2012. http://www.ine.es/prensa/np770.pdf. [ Links ]
34. Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett's esophagus. Am J Gastroenterol 2008;103:292-300. DOI: 10.1111/j.1572-0241.2007.01621.x. [ Links ]
35. Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:872-8. DOI: 10.1158/1055-9965.EPI-05-0860. [ Links ]
36. Vaughan TL, Kristal AR, Blount PL, et al. Nonsteroidal anti-inflammatory drug use, body mass index, and anthropometry in relation to genetic and flow cytometric abnormalities in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev 2002;11:745-52. [ Links ]
37. Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst 2000;92:1472-89. DOI: 10.1093/jnci/92.18.1472. [ Links ]
38. Veugelers PJ, Porter GA, Guernsey DL, et al. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus 2006;19:321-8. DOI: 10.1111/j.1442-2050.2006.00602.x. [ Links ]
39. Thrift AP, Cook MB, Vaughan TL, et al. Alcohol and the risk of Barrett's esophagus: A pooled analysis from the International BEACON Consortium. Am J Gastroenterol 2014;109:1586-94. DOI: 10.1038/ajg.2014.206. [ Links ]
40. Cook MB, Shaheen NJ, Anderson LA, et al. Cigarette smoking increases risk of Barrett's esophagus: An analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology 2012;142:744-53. DOI: 10.1053/j.gastro.2011.12.049. [ Links ]
41. Anaparthy R, Gaddam S, Kanakadandi V, et al. Association between length of Barrett's esophagus and risk of high-grade dysplasia or adenocarcinoma in patients without dysplasia. Clin Gastroenterol Hepatol 2013;11:1430-6. DOI: 10.1016/j.cgh.2013.05.007. [ Links ]
42. Pohl H, Pech O, Arash H, et al. Length of Barrett's oesophagus and cancer risk: Implications from a large sample of patients with early oesophageal adenocarcinoma. Gut 2016;65:196-201. DOI: 10.1136/gutjnl-2015-309220. [ Links ]
43. Van der Burgh A, Dees J, Hop WC, et al. Oesophageal cancer is an uncommon cause of death in patients with Barrett's oesophagus. Gut 1996;39:5-8. DOI: 10.1136/gut.39.1.5. [ Links ]
44. Moayyedi P, Burch N, Akhtar-Danesh N, et al. Mortality rates in patients with Barrett's oesophagus. Aliment Pharmacol Ther 2008;27: 316-20. DOI: 10.1111/j.1365-2036.2007.03582.x. [ Links ]
45. Portale G, Peters JH, Hsieh CC, et al. Esophageal adenocarcinoma in patients < or = 50 years old: Delayed diagnosis and advanced disease at presentation. Am Surg 2004;70:954-8. [ Links ]
![]() Correspondence:
Correspondence:
Quetzalihuitl Arroyo-Martínez.
Gastroenterology Intercenter Unit.
Hospitales Universitarios Virgen Macarena-Virgen del Rocío.
Avenida Dr. Fedriani, s/n.
41009 Seville, Spain.
e-mail: quetzalihu.arroyo.sspa@juntadenadalucia.es
Received: 29-01-2016
Accepted: 29-03-2016











 texto en
texto en