My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 n.1 Madrid Jan. 2017
https://dx.doi.org/10.17235/reed.2016.4557/2016
Malnutrition risk questionnaire combined with body composition measurement in malnutrition screening in inflammatory bowel disease
Ágnes Anna Csontos1, Andrea Molnár2, Zsolt Piri1, Erzsébet Pálfi3 and Pál Miheller1
1Second Department of Medicine. Semmelweis University. Budapest, Hungary.
2Pathological Sciences, Health Science Research. School of PhD Studies. Semmelweis University. Budapest, Hungary.
3Department of Dietetic and Nutrition Sciences. Faculty of Health Sciences. Semmelweis University. Budapest, Hungary
ABSTRACT
The purpose of malnutrition screening is to predict the probability of a worse outcome due to nutritional factors. The Malnutrition Universal Screening Tool (MUST) can be used for screening in inflammatory bowel disease (IBD); however, it does not provide details about body composition. Our aim was to assess the body composition and combine this with the MUST method to screen risk of malnutrition and sarcopenia. A total of 173 IBD outpatients were enrolled in this cross-sectional study. The MUST scale indicated 21.4% of IBD patients to be at risk of malnutrition. A risk of sarcopenia was detected in 27.7%. However, one third of these patients were not considered to be at risk by their MUST score. Furthermore, Crohn's disease (CD) patients had a strongly unfavorable fat-free mass index (FFMI) value compared to ulcerative colitis (UC) patients, and these differences were significant among men (FFMI: 18.62 ± 2.16 vs 19.85 ± 2.22, p = 0.02, in CD and UC males, respectively). As sarcopenia is a relevant prognostic factor, the MUST method should be expanded to include body composition analysis to detect more IBD patients at risk of malnutrition and sarcopenia in order to start their nutritional therapy immediately.
Key words: Malnutrition screening. Bioelectrical impedance analysis. IBD.
Introduction
The European Society of Clinical Nutrition and Metabolism (ESPEN) defined malnutrition as a state resulting from lack of uptake or intake of nutrition leading to altered body composition (decreased fat-free mass and body cell mass), resulting in diminished physical and mental function and impaired clinical outcome from disease (1).
Inflammatory bowel disease (IBD) is a chronic, systemic autoimmune disease involving chronic inflammation of the digestive tract. Reduced nutrition absorption, inadequate dietary intake, and chronic inflammation expose the patient to catabolic effects. This leads to an increased risk of malnutrition, including loss of muscle mass (2,3). Long-standing malnutrition may comprise sarcopenia, which is associated with decreased chances of survival, worse clinical outcome such as increased rate of postoperative infections or complications (4,5), increased toxicity to antitumor therapies in gastroenterological malignancies (6,7), and diminished quality of life in IBD patients (8). The development of sarcopenia is a common issue in IBD (9), therefore assessment of the nutritional status and screening of sarcopenia risk are important parts of IBD patient care. Using a suitable method in the appropriate time may help the practitioner screen potentially at risk patients at an early stage and introduce individualized nutrition therapy if necessary. The maintenance of an adequate nutrition therapy may reduce sarcopenia and it has the potential to improve well-being and the disease outcome.
According to current ESPEN Guidelines for Nutrition Screening (10), all hospitalized patients should be screened regularly for malnutrition with a validated tool (11-14). Malnutrition screening offers a simple and rapid process conducted by nurses or healthcare teams or patients (as self-screening) (15). We used the Malnutrition Universal Screening Tool (MUST) questionnaire to screen the risk of malnutrition. It is a quick test consisting of three questions: actual body mass index (BMI), weight loss, and presence of acute disease with regard to the nutritional intake. Although it is very simple, its main disadvantage is that it does not consider altered body composition. Despite having a normal BMI and a relatively low chance of being at risk, a person can be malnourished if the body fat-muscle ratio is abnormal. Patients with a very different distribution of fat mass and fat-free mass can have the same BMI value, and hence the risk of sarcopenia may not be detected. As the ESPEN states, the diagnosis of malnutrition should be based on either a low BMI (< 18.5 kg/m2) or the combination of weight loss together with either reduced BMI or a low fat free mass index (FFMI) (16), whilst sarcopenia is diagnosed if low FFMI is associated with a reduction in measured documented muscle strength or low performance (17).
There are several methods for measuring body composition; dual X-ray absorptiometry (DEXA), computed tomography (CT) and bioelectrical impedance analysis (BIA) are currently the most frequently used in clinical practice. These methods are able to indicate the possible tissue loss by distinctly analyzing the two major body components: fat-free mass and fat mass. The BIA is based on the characteristics of hydrated tissues conducting electricity. The measurement allows the estimation of total body water distribution, and thereby assesses body composition (18). This easy-to-use method has numerous advantages, such as reproducibility and the lack of ionizing radiation, and it also enables the monitoring of the effect of nutritional therapy. Our aim was to examine the clinical relevance of the two methods in malnutrition screening at the same time. We wanted to determine what proportion of the patients potentially at risk we miss when using only the MUST questionnaire and, furthermore, if there is any clinical relevance to involve BIA in first line screening process.
Agnes Anna Csontos and Andrea Molnár are equal authors of this manus-cript.
Methods
Participants
A total of 173 consecutive IBD patients (126 with CD and 47 with ulcerative colitis [UC]) were included in the study from September until December 2014.
Participants, who agreed to be included, were over 18 years of age and were outpatients of our tertiary IBD center.
The basic primary inclusion criteria were as follows: the subjects were diagnosed with IBD according to the Lennard-Jones criteria (19), they were able to adhere to the study protocol (i.e. suitable mobility to step up the InBody tool and to hold the hand electrodes), and their body mass index was from 16 kg/m2 to 34 kg/m2.
Exclusion criteria were tube or parenteral feeding, extremely low or high body mass index (< 16 kg/m2 or > 34 kg/m2), and other chronic or malignant diseases. Patients suffering from thyroid or other endocrine dysfunction were also excluded from this study. Due to the rapid and permanent effects of corticosteroid therapy to water and mineral metabolism, steroid dependent patients were also excluded from this study. BIA measurement was contraindicated for patients with defibrillation, cardiac pacemaker devices, or any metal implants. Patients with limb edema or notable ascites were also excluded to avoid inaccurate measurement due to water and electrolyte imbalances.
Design and data collection
UC and CD were divided into subgroups based on the Montreal classification (20). Further disease specific information and physical characteristics were collected from hospital files and from the patients during their visits to our outpatient department. By location, UC patients were divided into two groups (pancolitis or left-sided/distal colitis) based on the last endoscopic findings. Disease activity was defined by the partial Mayo score (pMayo) (21). Patients with CD were divided into two groups based on whether any small bowel involvement was found during the previous endoscopic or imaging (CT, magnetic resonance imaging [MRI]). Three categories were defined based on CD behavior: inflammatory, stenosing, and penetrating type. Crohn's disease activity index (CDAI) and perianal disease activity index (PDAI) were used to determine disease activity (22).
To evaluate malnutrition risk, we used MUST according to the ESPEN guidelines (10). MUST formulates a risk of malnutrition score based on current body mass index (0 points if BMI is > 20 kg/m2; 1 if BMI is in the range of 18.5-20 kg/m2; 2 if BMI is < 18.5 kg/m2), known weight loss (0 points if weight loss is < 5%; 1 if between 5-10%; 2 if weight loss is > 10%), and the presence of acute disease or no nutritional intake for FIVE days (2 points if either of them applies). Overall risk of malnutrition is determined from the sum of the points as follows: 0 = low risk; 1 = medium risk; and 2-6 = high risk.
Body composition was measured by the InBody 720 body analyzer device manufactured by Biospace. InBody 720 uses the segmental BIA method to examine the body as five cylinders (four limbs and the trunk) and measures impedance in these parts separately. It uses electrical currents at various frequencies (1-1,000 kHz) in order to measure electrical impedance and to derive the amount of extra- and intracellular water content in turn. Each patient was measured when fasting, after urination, and undressed except for underwear. All jewelry and wristwatches were removed before the measurement. Various parameters, including body weight, body mass index (BMI), body fat mass (BFM), fat-free mass (FFM), skeletal muscle mass (SMM), skeletal lean mass (SLM), total body water (TBW), mineral content, and body cell mass (BCM) were automatically calculated. According to the ESPEN recommendations, FFM and BFM were calculated for all participants and their respective indices were compared against reference data (23). Analogous to BMI, these indices were calculated as body composition parameters given in kg divided by the height in square meters; this transformation facilitates the interpretation of body composition variables regardless of height (24).
Sarcopenia can be considered as "primary" (or age-related) when no other cause is evident except for ageing itself, while it can be considered as "secondary" when one or more other causes are evident. In connection with IBD, we focused on secondary sarcopenia. According to the ESPEN recommendations, we defined the risk of sarcopenia when the fat-free mass was low, defined by FFMI ≤ 17 kg/m2 for men and ≤ 15 kg/m2 for women (16,25). As our study did not include a handgrip measurement to detect muscle function, we only detected a reduction of FFMI, which is called pre-sarcopenia, considered to be a potentially at risk state.
Ethical considerations
The study was approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics Committee (TUKEB number: 255/2013), and it was performed in accordance with the Declaration of Helsinki. Every patient matching the inclusion criteria agreed to participate and gave informed consent.
Data analysis
For our calculations the SPSS statistics v22.0 software was used. Paired and independent sample Student's t-tests and Pearson's correlations were applied. One-way analysis of variance (ANOVA) was performed for the comparison of means of continuous variables and normally distributed data; categorical variables were assessed by a Chi-squared or Fisher's exact test. Concordance between MUST and body composition metric data (FFMI and BMI) was calculated by analysis of variance, as MUST was categorical, whilst the body composition was a numerical variable. Cohen's kappa was also calculated with categorical variables based on the level of FFMI risk categories. The vertical part of the established 2x2 contingency table showed if a patient was at risk of low FFMI, while the horizontal part showed MUST risk.
Results are shown as mean ± standard deviation (SD). The level of significance was p < 0.05.
Results
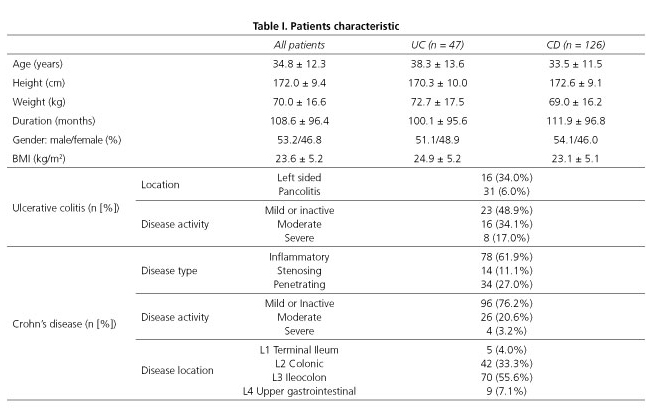
A total of 173 IBD patients were included in the study; 126 (72.8%) of them suffered from CD, while 47 (27.2%) were UC patients. Mean age was 34.8 ± 12.3 years. Major anthropometrical values were similar in the two patient groups (Table I).
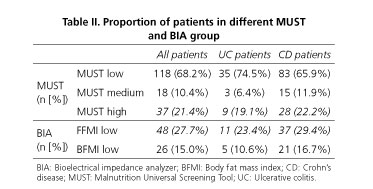
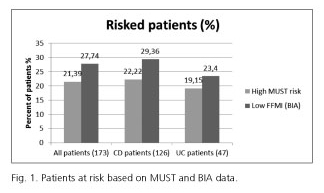
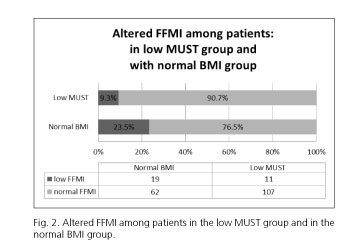
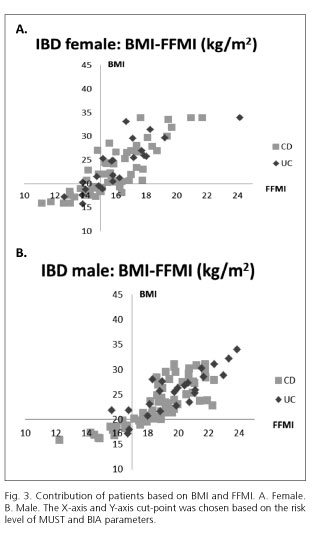
MUST indicated 37 (21.4%) while BIA (considering FFMI) indicated 48 (27.8%) patients to have alarmingly low parameters (Table II and Fig. 1). When comparing the body composition results in different MUST groups, we found that 11 (9.3%) patients in the MUST-based low-risk-of-malnutrition group had alarmingly low FFMI values, thus indicating a risk of sarcopenia (Fig. 2).
According to the concordance analyses, we found a modest relationship between MUST and BIA methods (with metrics data: BMI = 33.5% [p < 0.0001], FFMI = 29.2% [p < 0.0001]; categorical variables: Cohen's kappa = 0.53 [95% CI: 0.39-0.67]). We observed that 12.1% of all patients had a low MUST risk, while they were already malnourished based on FFMI.
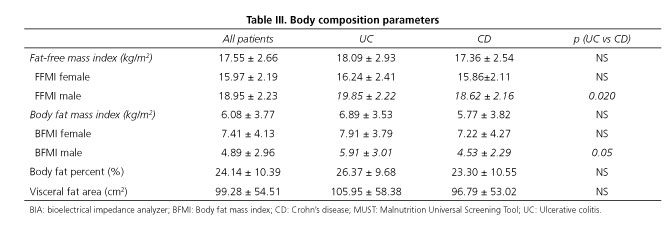
In our study 92 (53.2%) patients were male and 81 (46.8%) were female. We found no difference between the rate of being underweight or at risk of being malnourished among genders either in BMI or in MUST scores. Among women, there was no significant difference between the mean of BIA parameters in CD vs UC patients, whilst men suffering from CD had a significantly lower body composition index than men with UC (Table III).
BMI of CD patients differed significantly compared to UC patients (23.12 ± 5.11 vs 24.98 ± 5.20, p = 0.036, respectively). However, no significant differences were found in the number of patients categorized into different groups based on the MUST scale (Table II). CD patients had tendentiously lower scores in body composition parameters compared to UC patients (Table III).
Among UC patients, we found that the rate of malnutrition risk was 19.1% (n = 9), and an even higher proportion of at risk patients were detected by low FFMI based on body composition analysis 23.4% (n = 11). Measured body composition parameters were evaluated in subgroup analyses by the extensiveness of the disease and actual activity. According to our findings, the extent of the disease did not affect the body composition results significantly. We observed a weak positive correlation between the disease activity defined by pMayo score and FFMI (r = -0.316).
Among CD patients, the rate of being at risk of malnutrition was found to be 22.2% (n = 28) calculating MUST scores and 29.4% (n = 37) based on FFMI. Location, type, and disease activity were examined in a subgroup analysis. A higher proportion of small bowel involvement CD patients were underweighted by BMI than those with colonic disease (14.3% vs 4.0%), and they had more unfavorable body composition results as well. Small bowel involvement seems to be a potential risk factor in CD as significant differences were found in FFMI (16.91 ± 2.41 vs 18.24 ± 2.56, p = 0.05) and body fat mass index (BFMI, 5.08 ± 2.93 vs 7.15 ± 4.91, p = 0.004), respectively. However, the ratio of MUST low, medium, and high risks did not differ significantly between these two groups: the corresponding percentages were 61.9%, 14.3%, and 23.8% in low bowel involvement, and 73.8%, 7.1%, and 19.0% in colon involvement, respectively. Malnutrition risk and main BIA parameters were compared in patients with inflammatory, stenosing, and penetrating type of CD. None of them showed any statistically significant differences according to the disease behavior. However, patients with the stenosing type (n = 14) showed the worst nutritional status according to FFMI (42.9%, n = 6), or highest risk according to the MUST scale (57.1%, n = 8). No significant difference was observed regarding body composition parameters between patients with mild or moderate disease. Neither the duration of the disease nor the actual disease severity correlated with the body composition parameters.
Discussion
Appropriate assessment and follow-up of the nutritional status of IBD patients have gained more attention recently, but there is a lack of data regarding the suitable methods. Weight measurement based methods and questionnaires like MUST can be an important step in the screening of malnutrition risk. Low FFMI value was also defined by the ESPEN as a marker of risk of being malnourished, as not only body weight changes, but also altered body composition is a potential risk factor for worst outcome of the disease, and poorer quality of life in chronic disorders. In fact, even with an apparently normal BMI body composition can be altered.
Numerous diagnostic methods have been introduced to measure nutritional status. Based on the definition of malnutrition, its identification requires methods that simultaneously measure FFM and BFM. DEXA is a reproducible reference method for the clinical practice, although it has a high cost, X-ray exposition, low accessibility, a need for trained operators, and it cannot be performed at the bedside. CT can assess FFM by regional analysis of the third lumbar vertebra. The difficulties in this technique are similar to DEXA's. In contrast, BIA is an easy-to-use, non-invasive, relatively inexpensive method, which does not use ionizing radiation. A limitation of the BIA method is that it is not accurate during dehydration or over-hydration (26). For patients in the reproductive age group, as the significant majority of IBD patients are, a method without ionizing radiation should be the first choice in our opinion.
Nutritional status of IBD patients is particularly relevant as these patients are potentially at risk of malnutrition. Symptoms of acute disease (such as diarrhea, malabsorption, and decreased dietary intake) and the presence of chronic inflammation may lead to malnutrition and altered body composition as well. Bryan et al. recently reviewed the corresponding literature comparing the body composition of IBD patients and healthy controls (27). However, the number of patients was relatively low in the studies included; besides, the methodology of the measurements was heterogeneous as they used different DEXA and BIA instruments. Data of IBD patients (631 CD and 295 UC patients) were analyzed in this review. Statistically significant reduction in BMI was reported in 37% of CD and 20% of UC patients; reduced FFMI in 28% of CD and 13% of UC patients. Valentini et al. compared 144 IBD patients' nutritional status and body composition. They found most patients (74%) to be well nourished according to the subjective global assessment and BMI; however, body composition analysis demonstrated a decrease in body cell mass (28).
According to our results, the rates of patients at risk defined by the MUST score and by FFMI from the BIA measurements were notably different: while MUST indicated a risk of malnutrition for about 1/5 of our patients (21.4%), BIA indicated a potential risk of sarcopenia for more than 1/4 of them (27.7%). Our findings suggest that BMI-based questionnaires like MUST might miss a valuable proportion of IBD patients at risk of malnutrition. Body weight and changes in weight are still the main pillars of the MUST calculation, but they do not carry enough information about the composition of the body. According to our findings, 23.5% of the patients who were in the normal BMI range had alarmingly low FFMI, and a further 9.3% of the patients had decreased FFMI although being categorized to have low risk of malnutrition by the MUST scale. These results indicate that without BIA measurement we would miss nearly a quarter of the patients with normal BMI and every 10th patient in the low MUST category, although they are at risk of sarcopenia.
When combining the MUST and BIA methods, we found that 12.1% of the patients that had relatively low MUST scores already had altered body composition. These results are not surprising as MUST was not created to replace body composition measurement. However, our study seems to confirm and emphasize that the BIA method would have an adequate role in the primary screening process. By measuring FFMI, we may identify more patients with poor nutritional status, and it may also indicate the possibility of sarcopenia as the major risk factor for unfavorable clinical outcomes in chronic diseases. The results also warn us not to fall into the trap of just measuring body composition of patients at risk by MUST. Otherwise we would miss a relatively high proportion of patients who already should have nutritional support. Early diagnosis allows the establishment of a proper and individual nutritional plan under the control of a dietician. Nutritional therapy should be introduced forth with if the patient is deemed to be at risk from malnutrition questionnaires or if low FFMI (< 15 kg/m2 for females, < 17 kg/m2 for men) is detected (16). Monitoring body composition regularly helps to personalize nutritional plans and reduce the risk of malnutrition and sarcopenia, resulting in a better quality of life for our patients (29). IBD patients therapy needs a multidisciplinary approach and individually tailored diagnostic and therapeutic decisions at the same time. Combined use of MUST and BIA allows the dietician and the gastroenterologist to comply with all these principles. Although the examined population was fairly heterogeneous, the results of the subgroup analyses reveal the importance of body composition analyses of the vulnerable part of IBD patients. CD patients showed a higher risk of being malnourished than UC patients, furthermore stenosing CD type seemed to be a potential risk factor, as these patients possessed tendentiously worse body composition parameters. The findings suggest that CD patients, especially those with the stenosing type, need greater attention in the clinical practice. A significant difference was found in body composition parameters between patients categorized into different MUST categories in our study. These data indicate that MUST provides a reliable screening function for clinical use. However, its sole use might pose a risk of underdiagnosing the rate of unfavorable nutritional status in IBD patients. Furthermore, the most important limitation of the exclusive use of MUST as a screening tool is that it is not suitable for monitoring nutrition therapy. As all aspects of IBD patient care are leading towards personalized medicine, we believe that BIA could be a reasonable method to evaluate the risk of malnutrition, as well as to diagnose the risk of sarcopenia.
In the future, there may be a need to define also age and disease-specific, percentile-based thresholds, which can simplify the practitioner's screening tasks (30). In our view, malnutrition screening by MUST should be extended with body composition analysis by BIA, especially in IBD patients. We suggest combining them in patients suffering from IBD.
Limitations of the study
A limitation of our study is the relatively heterogeneous population and low number of cases in the subgroup analysis. Further studies should increase the number of participants to have far-reaching clinical implications. Although our study was conducted in a referral center for IBD, our outpatient population is quite diverse and comes from different parts of the country. We acknowledge that it may not be as representative as a multicenter survey.
Conclusions
According to our findings, the MUST scale is an optional and recommended part of outpatient care, and it is a useful tool to screen for the risk of malnutrition. However, it still does not provide sufficient information to select and follow the potentially at risk IBD patients in outpatient care. Body composition analysis, like BIA, should be implemented along with MUST in the clinical practice to optimize screening.
Acknowledgements
We would like to express our warm thanks to Ildikó Kovács for providing the BIA device, William Gesztes for providing language help as a native English speaker and to Tamás Ferenci for the help in statistical analyses.
References
1. Sobotka L. ESPEN Book - Basics in Clinical Nutrition. Prague: Publishing House Galén; 2011. [ Links ]
2. Rocha R, Santana GO, Almeida N, et al. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br J Nutr 2009;101:676-9. DOI: 10.1017/S0007114508032224. [ Links ]
3. Mijac DD, Jankovic GL, Jorga J, et al. Nutritional status in patients with active inflammatory bowel disease: Prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med 2010;21:315-9. DOI: 10.1016/j.ejim.2010.04.012. [ Links ]
4. Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. British Journal of Cancer 2012;107: 931-6. DOI: 10.1038/bjc.2012.350. [ Links ]
5. Zhang T, Cao L, Cao T, et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn's disease undergoing bowel resection. J Parenter Enteral Nutr 2015. DOI: 10.1177/0148607115612054. [ Links ]
6. Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutrition and Cancer 2014;66:583-9. DOI: 10.1080/01635581.2014.894103. [ Links ]
7. Cousin S, Hollebecque A, Koscielny S, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Investigational New Drugs 2014;32:382-7. DOI: 10.1007/s10637-013-0053-6. [ Links ]
8. Norman K, Kirchner H, Lochs H, et al. Malnutrition affects quality of life in gastroenterology patients. World Journal of Gastroenterology 2006;12:3380-5. DOI: 10.3748/wjg.v12.i21.3385. [ Links ]
9. Schneider SM, Al-Jaouni R, Filippi J, et al. Sarcopenia is prevalent in patients with Crohn's disease in clinical remission. Inflammatory Bowel Diseases 2008;14:1562-8. DOI: 10.1002/ibd.20504. [ Links ]
10. Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21. DOI: 10.1016/S0261-5614(03)00098-0. [ Links ]
11. Stratton RJ, Hackston A, Longmore D, et al. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the "malnutrition universal screening tool" ("MUST") for adults. Br J Nutr 2004;92:799-808. DOI: 10.1079/BJN20041258. [ Links ]
12. Vellas B, Villars H, Abellán G, et al. Overview of the MNA - Its history and challenges. J Nutr Health Aging 2006;10:456-63; discussion 463-5. [ Links ]
13. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment Short-Form (MNA-SF): A practical tool for identification of nutritional status. J Nutr Health Aging 2009;13:782-8. DOI: 10.1007/s12603-009-0214-7. [ Links ]
14. Olivares J, Ayala L, Salas-Salvado J, et al. Assessment of risk factors and test performance on malnutrition prevalence at admission using four different screening tools. Nutr Hosp 2014;29:674-80. DOI: 10.3305/nh.2014.29.3.7120. [ Links ]
15. Sandhu A, Mosli M, Yan B, et al. Self-screening for malnutrition risk in outpatient inflammatory bowel disease patients using the Malnutrition Universal Screening Tool (MUST). J Parenter Enteral Nutr 2016;40: 507-10. DOI: 10.1177/0148607114566656. [ Links ]
16. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis - Part I: Review of principles and methods. Clin Nutr 2004;23:1226-43. DOI: 10.1016/j.clnu.2004.06.004. [ Links ]
17. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 1989;170:2-6;discussion 16-9. DOI: 10.3109/00365528909091339. [ Links ]
18. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;(Suppl A):5A-36A. DOI: 10.1155/2005/269076. [ Links ]
19. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J Crohns Colitis 2012;6:965-90. DOI: 10.1016/j.crohns.2012.09.003. [ Links ]
20. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis 2010;4:7-27. DOI: 10.1016/j.crohns.2009.12.003. [ Links ]
21. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis - Part II: Utilization in clinical practice. Clin Nutr 2004;23:1430-53. DOI: 10.1016/j.clnu.2004.09.012. [ Links ]
22. Kyle UG, Schutz Y, Dupertuis YM, et al. Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition 2003;19:597-604. DOI: 10.1016/S0899-9007(03)00061-3. [ Links ]
23. Franssen FM, Rutten EP, Groenen MT, et al. New reference values for body composition by bioelectrical impedance analysis in the general population: Results from the UK Biobank. Journal of the American Medical Directors Association 2014;15:448e1-6. DOI: 10.1016/j.jamda.2014.03.012. [ Links ]
24. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin Nutr 2014. DOI: 10.1016/j.clnu.2014.03.007. [ Links ]
25. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr 2015;34:335-40. DOI: 10.1016/j.clnu.2015.03.001. [ Links ]
26. Thibault R, Genton L, Pichard C. Body composition: Why, when and for who? Clin Nutr 2012;31:435. E-pub: 2012 Jan 31. DOI: 10.1016/j.clnu.2011.12.011. [ Links ]
27. Bryant RV, Trott MJ, Bartholomeusz FD, et al. Systematic review: Body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther 2013;38:213-25. E-pub: 2013 Jun 14. DOI: 10.1111/apt.12372. [ Links ]
28. Valentini L, Schaper L, Buning C, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition 2008;24:694-702. DOI: 10.1016/j.nut.2008.03.018. [ Links ]
29. Kudsk KA, Muñoz-del-Río A, Busch RA, et al. Stratification of fat-free mass index percentiles for body composition based on National Health and Nutrition Examination Survey III Bioelectric Impedance Data. J Parenter Enteral Nutr 2015. DOI: 10.1177/0148607115592672. [ Links ]
30. Wedrychowicz A, Zajac A, Tomasik P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J Gastroenterol 2016;22:1045-66. DOI: 10.3748/wjg.v22.i3.1045. [ Links ]
![]() Correspondence:
Correspondence:
Ágnes Anna Csontos.
Second Department of Medicine.
Semmelweis University.
46 Szentkirályi street.
Budapest, Hungary
e-mail: csontosagnesanna@gmail.com
Received: 27-07-2016
Accepted: 07-10-2016