Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.6 Madrid Jun. 2017
https://dx.doi.org/10.17235/reed.2017.5035/2017
SPECIAL ARTICLE
Quality indicators in digestive endoscopy: introduction to structure, process, and outcome common indicators
Indicadores de calidad en endoscopia digestiva: introducción a los indicadores comunes de estructura, proceso y resultado
Julio López-Picazo1, Fernando Alberca-de-las-Parras2, Antonio Sánchez-del-Río3, Shirley Pérez-Romero1, Joaquín León-Molina4 and Javier Júdez5, on behalf of the SEPD's Taskforce on Endoscopy Quality Indicators
1Service of Health Care Quality. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia, Spain.
2Endoscopy Unit. Service of Digestive Diseases. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia, Spain.
3Service of Digestive Diseases. Hospital San Juan de Dios. Santa Cruz de Tenerife, Spain.
4Service of Digestive Diseases. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia, Spain.
5Department of Knowledge Management. SEPD. Madrid, Spain
ABSTRACT
The general goal of the project wherein this paper is framed is the proposal of useful quality and safety procedures and indicators to facilitate quality improvement in digestive endoscopy units. This initial offspring sets forth procedures and indicators common to all digestive endoscopy procedures.
First, a diagram of pre- and post-digestive endoscopy steps was developed.
A group of health care quality and/or endoscopy experts under the auspices of the Sociedad Española de Patología Digestiva (Spanish Society of Digestive Diseases) carried out a qualitative review of the literature regarding the search for quality indicators in endoscopic procedures. Then, a paired analysis was used for the selection of literature references and their subsequent review.
Twenty indicators were identified, including seven for structure, eleven for process (five pre-procedure, three intra-procedure, three post-procedure), and two for outcome. Quality of evidence was analyzed for each indicator using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) classification.
Key words: Quality indicators. Endoscopy. Digestive system.
RESUMEN
El objetivo general del proyecto en el que se inscribe este trabajo es proponer procedimientos e indicadores de calidad y seguridad útiles para facilitar la mejora de la calidad en unidades de Endoscopia Digestiva. En este primer resultado se proponen procedimientos e indicadores comunes a las pruebas de endoscopia digestiva.
Primero, se ha diseñado un diagrama de los pasos previos y consecutivos a la realización de la endoscopia digestiva.
Un grupo de expertos en calidad asistencial y/o endoscopia, bajo el amparo de la Sociedad Española de Patología Digestiva, han realizado una revisión cualitativa de la literatura haciendo referencia a la búsqueda de indicadores de calidad en los procedimientos endoscópicos. Posteriormente, por un procedimiento de análisis por pares se ha hecho la selección y análisis de la literatura seleccionada.
Se ha identificado un total de 20 indicadores, de los cuales siete son de estructura; once, de proceso (cinco de preprocedimiento, tres de procedimiento y tres de posprocedimiento); y dos, de resultado.
Se ha analizado la calidad de la evidencia de cada uno de ellos aplicando la clasificación utilizada en GRADE (Grading of Recommendations Assessment, Development and Evaluation).
Palabras clave: Indicadores calidad. Endoscopia. Aparato digestive.
Introduction
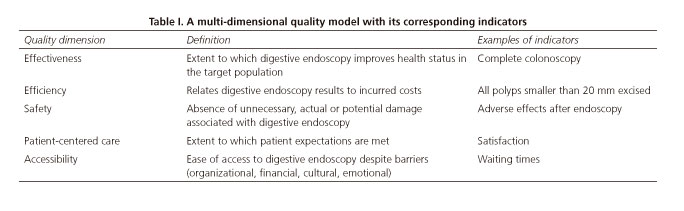
It is well known that, in order to assess and improve care quality, the latter term must be first defined (1) and its dimensions identified (2), including effectiveness, efficiency, safety, accessibility, and patient-centered service (Table I). Once these dimensions, their requirements for each undertaken activity, and the target results are established, designing things so that they may be completed at the first go (for instance, by describing procedures to be implemented and their requirements) significantly facilitates work.
In order to acknowledge the quality level achieved, and to start making improvements if needed, consistent information is necessary on the most relevant aspects of the care provided, which may be summarized as indicators. Quality indicators may be divided up into three categories: "structure", "process" and "outcome or results" (3). "Structure" includes all things related to the stable attributes wherein care is provided, both material and organizational; "process" includes all things that are done for patients and the skills involved; and "outcome" denotes any health status changes that may be attributed to the received care, as well as patient satisfaction.
In this setting, the Sociedad Española de Patología Digestiva (SEPD) understood that quality indicators associated with digestive endoscopy procedures should be analyzed and assessed, both overall and specifically (the goal of this first report) for the three main procedures in terms of volume and impact: gastroscopy, colonoscopy, and endoscopic retrograde cholangio-pancreatography (ERCP). In a second stage, echoendoscopy and balloon enteroscopy will also be analyzed. The reason is none other than to safeguard practice quality in the field of gastroenterology in Spain by providing quality indicators adapted to our setting and according to evidence levels.
The overall goal of this project is to suggest quality and safety procedures and indicators useful to facilitate quality improvement in digestive endoscopy units. This paper puts forth procedures and indicators common to the various digestive endoscopy tests.
Methods
The study was structured in two clear-cut stages. First, a multidisciplinary task force was set up and headquartered at the Hospital Clínico Universitario Virgen de la Arrixaca (HCUVA), which reviewed the literature and the design of diagnostic esophagogastroscopy, colonoscopy, and ERCP procedures. In a second stage proposals were reviewed and discussed by an expert panel selected by the SEPD until a final version was produced. Then data sheets were developed for each proposed indicator to assess these procedures.
Search strategies and study selection
Two broad, systematic literature searches were performed. The first one was for clinical practice guidelines (CPGs), the second was for original and review papers. CPGs related to digestive endoscopy were obtained from three international sources (Agency for Healthcare Research and Quality [AHRQ], National Institute for Health and Care Excellence [NICE] and Scottish Intercollegiate Guidelines Network [SIGN]) and one Spanish source (GuíaSalud), as well as from reviews in the web sites of the main endoscopy and gastroenterology societies (American Society for Gastrointestinal Endoscopy [ASGE], American Gastroenterological Association [AGA], European Society of Gastrointestinal Endoscopy [ESGE], Sociedad Española de Endoscopia Digestiva, SEED [SEPD], and Asociación Española de Gastroenterología [AEG]). Originals were searched in the Web of Knowledge (WOK), PubMed, and Cochrane databases using the following strategy. All documents were selected that were published between January 1, 2006 and August 10, 2016 and included any of the following descriptors: [digestive endoscop*, gastrointestinal endoscopy, colonoscop*, gastroscop*, oesophagoscop*, endoscopic retrograde cholangiopancreatography] and [informed consent, quality, safety, (security), assessment, assurance, indicators, criteria]. (Active filters: clinical trial, controlled clinical trial, meta-analysis, randomized controlled trial, review, guideline, practice guideline, publication date from 2006/01/01 to 2016/08/10; humans; adults; language: English, Spanish).
A review of the references included in the selected, analyzed originals, published guidelines and meta-analyses was also performed, and previously overlooked references of interest were selected.
Once the search protocol was completed, eligible papers were split in two groups (n1: other procedures [non-colonoscopy], and n2: colonoscopy). The documents selected were reviewed and analyzed separately by two reviewers. Each pair of reviewers screened their studies using any of the following criteria:
-The document includes recommendations on appropriate preparation, execution, and follow-up.
-The document includes or suggests quality indicators for structure, process or outcome.
Studies selected by just one reviewer were collated by the rest in order to decide their final selection status.
A table listing information on structure, process or outcome indicators, and whether such information was explicit, was developed to uniformly examine each document. Furthermore, the table included the type of each referenced study (clinical trial, observational study, meta-analysis, etc.), which was identified as pertaining to endoscopy in general, gastroscopy, colonoscopy or ERCP, with the rest of procedures being set aside. Document suitability for the intended goal (analysis of endoscopy-related quality indicators) was also discussed.
Endoscopy procedure design
Based on the collected literature and the authors' experience, the activities needed for each procedure were recorded and sorted out. In the case of procedures common to all endoscopic studies, the logical differences in structure, function, and organization among clinical digestive endoscopy units restrict development down to a minimum. Similarly, a description of specific techniques to be used in selected situations was excluded, as it was not contemplated in the ongoing work. Results were plotted in flow charts or parallel line diagrams. Proposals put forth by the group were reviewed and discussed by an expert panel selected by the SEPD until a final version was produced.
Construction of indicators
In order to obtain valid indicators, the quality of the available knowledge was assessed regarding the activities involved in the procedures and the documents selected after the search. To this end, the quality of knowledge grading scheme available within the GRADE model was used. In the GRADE system the quality of "evidence" (this term will be hereafter used without quotation marks to denote the "best proof-based knowledge available") is initially classified as high or low, according to its origin in experimental or observational studies; then, quality is rated as high, moderate, low or very low depending on a number of considerations regarding items that may downgrade or upgrade baseline quality (4,5).
To ensure reliability and to facilitate the estimation of the chosen indicators in clinical units, each of them is associated with a data sheet including: use environment (procedures wherein it is used); denomination; calculation formula; indicator type according to Donabedian's model (3); time relationship with test (pre-procedure, procedure, post-procedure); quality dimension involved; justification, exclusions, and clarifications; and supporting evidence level.
Results and discussion
Search results
A total of 617 references were identified in the various databases according to the designed search strategy. Once duplications were excluded, a total of 123 references were discarded when titles/abstracts were examined (wrong references, abstract only, obvious poor quality, unavailability, older than 2006, dealing with pediatric, veterinary or non-digestive endoscopic topic, language other than Spanish or English).
Post-hoc, 19 additional references were selected from the references found in other already published papers, reviews, meta-analyses, and clinical practice guidelines.
For the paired analysis, we examined a total of 513 papers (n1: 253; n2: 260) in full text format, including both randomized and non-randomized clinical trials, as well as 224 full articles on high-quality case series, reviews, and meta-analyses (n1: 117; n2: 107).
Results are plotted in figure 1.
Common procedures
Two common procedures were implemented: admission to unit on the day scheduled for endoscopy, and discharge from unit after the procedure. The inclusion of speed-up procedures and waiting list management was dismissed on the grounds mentioned under "Methods".
The admission procedure (Fig. 2) is intended to confirm that the patient is fully eligible for endoscopy, to ascertain the type of sedation that will be used, and to provide the necessary pre-procedural nursing care. It lasts from the time the patient presents to the endoscopy unit until endoscope insertion. It includes the following activities:
- Before entering the room:
• Confirm appointment and agenda availability (time/room) and mark as present.
• Confirm adequate preparation (see indicator below).
• Check out informed consent.
- In the endoscopy room:
• The endoscopist must:
- Confirm informed consent and clarify last-minute concerns.
- Open up the appropriate form within the medical record.
- Offer sedation if applicable, with its related informed consent.
- Confirm key history data such as allergies, use of antithrombotic drugs, or sedation contraindications.
• The nurse must:
- Insert a peripheral venous access, install a pulse oximeter, and provide oxygen through nasal prongs if sedation deeper than topical is required.
- Administer appropriate sedation.
The discharge from endoscopy unit process is intended to adequately document the procedure's course, findings, and potential incidences; to ensure the necessary care until the patient leaves the unit; and to prepare the endoscopy room for a new procedure. It lasts from endoscope withdrawal to patient leaving the unit (Fig. 3). It includes the following activities:
- Ancillary personnel must clean out the equipment and recondition the endoscopy room.
- The endoscopist must write a medical report (see indicator below) and make sure it is included in the patient's medical records.
- After patient transfer to the care room nurses must monitor vital signs, assess level of consciousness, and provide appropriate post-procedural care (see indicator below).
- Patients must receive a copy of the endoscopy report before leaving the unit.
Indicators
Twenty indicators have been included, of which seven refer to structure, two refer to outcome, and eleven are related to process. Table II lists all indicators used.
Structure indicators
A1. Valid informed consent

Providing patients with information regarding their therapy options and diagnostic choices, in an understandable manner, so that they may take part in the decision-making process involving their care, is both a duty health care professionals must fulfill (6) and a right guaranteed to patients by law (7). Hence, there is no doubt that informed consent forms are needed for digestive endoscopy procedures (8,9). These provide patients with information on the involved procedure so that an informed decision is made. Therefore, consent forms must meet formal quality requirements in order to observe legal regulations, promote readability, and improve decision making.
In this regard the criteria put forward by the Murcia Region EMCA program have proven useful in order to improve informed consent forms (10). Furthermore, it is also necessary to ensure the readability and understandability of these forms, as well as the validity of their contents (11,12). To achieve the former, the INFLESZ tool may be used, which is a validated scale to assess ease of comprehension for documents written in Spanish (scores of at least 55 are highly likely to provide the average population with an understandable text) (12), as well as other items (11). To achieve the latter it is key that texts and their supported evidence be up-to-date (13).
A2. Antithrombotic medication management plan

Although diagnostic endoscopies are usually considered to have a low bleeding risk, the same does not apply to some therapeutic procedures (8). In these patients the risk of thromboembolic complications associated with treatment discontinuation must also be assessed (14), and the timing of anticoagulant and/or antiaggregant drug withdrawal and reinitiation has to be decided upon, together with the need for monitoring during said periods (15). Because of this, several guidelines approach this subject in the context of digestive endoscopy (16), and several scientific societies recommend its inclusion among quality indicators (8).
About the minimal requirements an antithrombotic medication management plan should meet, it is suggested that validity criteria, a key aspect of clinical guidelines, be ascertained (17,18) and potentially assessed by checking out that recommendations include their strength and evidence level, and the plan includes an explicit validity and review period.
A3. Experienced endoscopist

An endoscopic procedure must reach its intended goal while minimizing the incidence of adverse events. Evidence suggests that endoscopist inexperience and insufficient training represent barriers to these ends (16,19,20).
Some scientific societies have established recommendations to assess expertise in endoscopy, which include a minimum of procedures performed to meet basic quality standards, as well as a minimum of procedures yearly (8,21,22).
Other factors to consider will depend upon the type of procedure and have to do with diagnostic or therapeutic yield and safety (23). Expertise in a given procedure does not qualify for other procedure types (20).
A4. Discharge plan

Trained nursing staff is usually responsible for patient recovery, including patient monitoring and the assessment of patient discharge criteria. The plan must also include a report on the procedure and its ensuing process, as well as patient instructions (food ingestion, forbidden activities within 24 hours, etc.) and follow-up assessments, including potential complications and their recognition (23,24).
The duration and frequency of monitoring should be individualized according to sedation level and patient health status, and range from 30 minutes to two hours. In this respect level of consciousness as assessed by response to verbal stimuli, vital signs (heart rate, respiratory rate, oxygen saturation), and level of pain must be monitored and recorded regularly until their return to normal (20,21,25).
Standard tools such as the modified Aldrete scale (22) are available to assess recovery after sedation (respiration, oxygen saturation, blood pressure, level of consciousness, activity). At discharge, patients must be fully aware and oriented with stable, normal vital signs (heart rate, respiratory rate, blood pressure, oxygen saturation), with a score of at least 9 points in the modified Aldrete scale (23,26).
A5. Discharge report quality

Providing accurate, timely information on the procedure performed, succinctly including all relevant data, improves patient care. Both the ASGE (8,27) and the Canadian Association of Gastroenterology (CAG) (10,28,29) recommend the inclusion of selected items in the endoscopy report: date of procedure; patient identification; endoscopist and other staff identification; relevant history and physical examination data; conditions that may interfere with the procedure or with sedation; drug allergies; medications, particularly anticoagulant and antiplatelet drugs; anesthetic risk assessment (American Society of Anesthesiologists [ASA]) (28,30); proof of informed consent; endoscopic procedure; indication; type of endoscope; medication during the procedure (analgesia, anesthesia, sedation) (31) and during recovery (if applicable); anatomical extent of the procedure; barriers and restrictions encountered during preparation and procedure (9) (type, quality); samples obtained, including type, location and sampling technique; findings, with a detailed description of present/pertinent absent lesions; diagnosis, using as much as possible standardized, coded terminology (32); therapies administered and outcomes, complications and adverse events; recommended actions (new appointments, collection of results, etc.), and recommended care.
A6. Disinfection procedure for endoscopy equipment

While the risk of infection is low (one case per 1.8 million endoscopies), digestive endoscopy is an invasive procedure where infection may be transmitted, hence every effort to minimize it must be in place. A consistently, appropriately executed cleaning and disinfection procedure is effective to get rid of bacteria, mycobacteria, and viruses (33,34).
To ensure adequate endoscope disinfection three steps should be followed: mechanical cleaning, disinfection, and rinsing and drying.
Guidelines and consensus documents by different societies (35-48) recommend the following:
- Perform test to identify potential loss of tightness in channels or presence of inner disruptions that may impair disinfection.
- Suction out and submerge the endoscope in an enzymatic detergent solution.
- Carefully clean and brush the whole endoscope, including valves and channels, using an enzymatic detergent (49).
- Use a device-friendly disinfectant agent of proven efficacy such as 2% glutaraldehyde, 0.4-1% glutaraldehyde-phenate, or 0.2% peracetic acid (48,50,51).
- Submerge the endoscope in disinfectant filling up the channels. Exposure duration and temperature vary according to product (52).
- If an automatic machine is used, check it is properly connected and follow manufacturer instructions.
- Replace disinfectant after its activity period regardless of minimum effective concentration.
- Once disinfected, rinse the endoscope using preferably sterile water and dry all channels with air.
- The use of 70% alcohol, followed by further air drying, may enhance disinfection efficacy.
- Store the endoscope vertically in a well-ventilated area. Valves and instrument channel cap should be stored separately (9).
- The irrigation bottle and tubing must undergo "high-level" disinfection daily (53).
- Ancillary materials potentially in contact with blood must be sterilized following careful mechanical cleaning (54).
- If any material is stored under non-sterile conditions for over 48-72 hours, reprocessing is advised in view of contamination risks (55).
A7. Structural and functional characteristics of an endoscopy unit

The structural and functional requirements of endoscopy units may differ to a greater or lesser extent according to location and type (10,53,56).
In this context, all of them must secure protocols, equipment, and trained, experienced personnel to provide safe, effective conscious sedation.
Endoscopy unit accreditation is directly related to the ability to provide endoscopy training (22).
In Spain, a document granted by the Ministry of Health and Consumption (57) includes the structural and functional characteristics digestive endoscopy units must have according to their defined typology (Table III). It also points out that they must be fitted with computer systems capable of processing and storing images, and of facilitating the writing of standard reports.
B1. Appropriate indication

The test must be indicated when the information it may yield or the therapy it may provide will improve the patient's outcome with a good risk-benefit ratio, as evidence suggests this improves cost-effectiveness (8,10,53,58) and provides a reference for legal claims (30). A list of gastroscopy indications was published by the ASGE and updated in 2012 (58). The European Panel on the Appropriateness of Gastrointestinal Endoscopy (EPAGE) II criteria are used for colonoscopy (59-64). Consensus documents also provide data for ERCP (58,65-67).
When a test is appropriately indicated, a higher rate of relevant diagnoses is obtained (68-72).
B2. Signing the informed consent form

Providing patients with information on their therapeutic and diagnostic options in an understandable way, so that it may be used to promote their involvement in the decision making process regarding their care (8), is both a duty for health practitioners and a right guaranteed to patients by law (7).
This indicator may be exempted in case of emergency. The signed ICF must meet formal quality requirements or be accredited by the institution where the test is performed or by its corresponding scientific society (see structure criteria) (9,10,12,53,73).
In any case, the decision to undergo endoscopy should not be made under duress, and the patient must acquiesce in writing by signing the consent form. The patient must be allowed time and opportunities to pose additional questions before making a decision (74). According to a recent systematic review (75), interventions to promote the consent process in patients undergoing surgery or other invasive procedures such as digestive endoscopy usually enhance comprehension.
B3. Clinical assessment

Assessing a patient's clinical status improves test safety. This implies that all risk factors must be considered to minimize complications during the procedure. Since many adverse effects during endoscopy are associated with sedation, it is advisable that individual risk for sedation be evaluated to adjust the regimen to be administered.
The following must be verified before the procedure (8,9,30,76,77):
- The patient has been informed about the procedure and has no concerns.
- Consent signed by both the patient and the physician.
- Scheduled preparation is appropriately followed.
- Fasting time as instructed.
- Antiplatelet or anti-inflammatory drugs (past seven days), or anticoagulants (past three or four days).
- Drug allergies.
- Removal of all metallic objects and dentures, when applicable.
- Issues in prior examinations, when present.
- The patient is accompanied and will not need to drive.
Another poorly clarified concept is the need to pause of the endoscopy team before a procedure in order to ensure the patient is aware of the procedure's reason, course and goals.
B4. Sedation plan

Sedation should be offered by levels to all patients according to their clinical status. The patient will choose one among the available options once adequately briefed, which is associated with greater satisfaction.
The level of sedation (from none to deep) to be used during the procedure must be recorded (53,78).
B5. Antithrombotic medication management

Diagnostic endoscopies are usually deemed to entail a low bleeding risk. This is not the case for some therapeutic procedures where a graded risk assessment applies (79). These patients must also have their risk for thromboembolic complications after therapy discontinuation assessed (8-10,14,16-18,80-82). Therefore, the timing for anticoagulant and/or antiaggregant discontinuation and reintroduction must be defined, as well as the need for monitoring during that period (79). Furthermore, decisions must be agreed with the patient (83).
C1. Graphical documentation

No studies have approached the effectiveness of including graphical documentation on the procedure, but it represents a universally accepted good practice requirement. Thus, the ASGE (8) and other guidelines (9,10,53,84,85) recommend that photographs be taken of the cecum as a quality parameter for colonoscopy (86). They are also recommended for other procedures since lesion's pictures enhance patient understanding and facilitate inter-consultations and second opinions (87).
C2. Sedated patient monitoring

Patient monitoring improves procedure safety but not clinical outcome.
According to the ASGE (8,26,78) and CAG (10), monitored parameters should include oximetry, heart rate, and blood pressure (the latter two at intervals no longer than five minutes), which help identify potentially life-threatening cardiovascular changes during sedation (53,88,89).
Evidence is insufficient to recommend capnography for patients sedated with propofol (9,26,90).
C3. Recording of immediate adverse events

Patient safety requires that damaging or potentially damaging events be identified and followed up (30,91). The recording of events emerging during the procedure or before leaving the endoscopy unit is therefore key in the effective implementation of programs to improve patient safety (30).
Efforts have been made to grade the severity of adverse events (92), and to establish a coherent classification (93). The following adverse events must be included:
1. Medication-related (94):
a) Need for cardiopulmonary resuscitation. Use of reversal medication, such as flumazenil and naloxone, to antagonize the sedative effects of benzodiazepines and opiates, respectively, which indicates excess sedation. Elective use to speed up recovery is inconsistent with better practice, and not recommendable in view of potential rebound sedation when the patient is no longer supervised (68,95,96).
b) Hypoxemia (< 85%). The risk of sequelae from hypoxemia is poorly understood, but an association with higher risk for late adverse events and longer recovery has been reported.
c) Hypotension (< 90/50 mmHg or ≤ 20% from baseline) or hypertension (> 190/130 mmHg or ≥ 20% from baseline) may trigger endoscopy termination, require direct intervention, have adverse consequences, and/or delay recovery.
d) Allergic reactions including laryngospasm/bronchospasm.
2. Procedure-related (97-99):
a) Perforation (100).
b) Immediate bleeding after polypectomy. Uncertain clinical outcome. Some authors suggest that even with immediate hemostasis significant complications may develop, this being a risk factor for subsequent bleeding events (101).
c) Need for admission or transfer to the Emergency Room for any procedure-emergent event.
d) Tube impaction, which may require surgery.
e) Persistent, severe abdominal pain requiring careful assessment to rule out perforation. In a randomized trial, 45% and 31% of patients undergoing colostomy experienced abdominal pain after one and six hours. While this pain usually subsides, in some patients it is persistent to the extent of requiring medical care. Pain from air retention during or after colonoscopy may be reduced when CO2 insufflation (102,103) or water immersion are used (104).
f) Instrument failures requiring a repeat procedure.
D1. Patient recovery

Recovery is usually supervised by trained nurses who perform the monitoring and assess discharge criteria.
The duration and frequency of the monitoring must be tailored to each patient according to sedation level and general health status (8,9), and should range from 30 minutes to two hours.
The level of consciousness as assessed by the response to verbal stimuli, together with vital signs (heart rate, respiratory rate, oxygen saturation) and pain level, must be regularly monitored and recorded until their return to normal (10).
Standard scales (modified Aldrete) (105,106) are available to assess recovery from sedation (breathing, oxygen saturation, blood pressure, level of consciousness, activity). Patients at discharge must be aware and oriented, with normal, stable vital signs (heart rate, breathing rate, blood pressure, oxygen saturation), with a score of at least 9 points on the modified Aldrete scale (31,107,108).
D2. Information on discharge

Information on discharge must include the procedure's course and findings, process follow-up (further appointments, reviews), and patient instructions, as well as potential adverse events and how to recognize them. This is known to reduce anxiety, to enhance recall of test conclusions and recommendations, and to improve patient adherence (8,9,30).
This is verified by ascertaining a high-quality report was written, discussed, and delivered before patient leave (10,27,31,53,109,110).
D3. Recording delayed adverse events

Patient safety requires the identification and follow-up of damaging or potentially damaging events (91). While the recording of adverse events occurring during the procedure is widespread, the recording of those developing after leaving the Endoscopy Unit is also key for the effective implementation of programs to enhance patient safety (15,30,94,97).
According to the CAG, adverse events that should be recorded include (93):
- Death within 30 days after the procedure.
- Unscheduled admission or emergency visit within 14 days after the procedure.
- Gastrointestinal bleeding within 14 days after the procedure.
- Infection, both acute and chronic.
- Symptomatic metabolic complication (hypoglycemia or hyperglycemia, electrolyte disorders). Colonoscopy requires colon cleansing with a laxative preparation. There is evidence of associated metabolic disorders such as hypokalemia, hyponatremia, hypocalcemia, and renal failure. Phosphate-containing preparations have been associated with acute phosphate nephropathy and their use is limited. Furthermore, preparation for colonoscopy may interfere with the management of conditions such as diabetes mellitus.
- During ERCP the development of procedure-induced pancreatitis depends upon the expertise of the endoscopist and the techniques performed.
E1. Incidence of adverse events

Indicators C3 and D3 refer to the recording of immediate and delayed events (30,89,94,97,107). The purpose of such recording is twofold: on the one hand, to know the magnitude, typology, and transcendence of adverse events emerging from digestive endoscopy procedures; and on the other, to assess their potential for prevention and to establish specific improvement actions.
E2. Perceived quality and patient satisfaction

A measure of care quality cannot be considered as fulfilled without an assessment of the patient perceived quality and patient satisfaction with the care received. In fact, the Institute of Medicine (IOM) highlights patient-centered care as one of the six major values of health care (108). Therefore, satisfaction measurements in the digestive endoscopy setting have been repeatedly proposed by authors and scientific societies alike (8,10,53,106,111). The above suggested measurement involves an 11-item (0-10) Likert scale that we feel better suited to Spanish context as compared to the 5- or 7-item scales usually seen in other settings and cultures. With this scale, useful measurements may be obtained to understand its evolution and set a ground for the analysis of conditioning factors such as the mean, median or percentage of excellent scores, the latter understood as the number of scores equal to or greater than 8 over the total of scores, as has already been tested in other environments (112,113).
However, measuring satisfaction is not enough. In order to implement an ongoing improvement approach and know where interventions are needed, conditioning factors must also be learned. Some derive from the other indicators suggested above. Others will have to be explored based on perceived quality items. In this respect, the Endoscopy Global Rating Scale (GRS) initiative, successfully developed in the United Kingdom, may represent a good starting point for their identification, as one dimension refers to perceived quality (114). GRS is a web-based assessment tool that provides statements requiring a yes or no answer, the usefulness of which has been tested in various settings and health systems (115,116). The six level-graded items of patient-centered care include equality and equity, opportunity (waiting times), ability to select dates (accessibility), privacy and dignity, post-procedural care, and capacity to generate return information to the endoscopy unit.
Acknowledgements
We are grateful to the SEPD's Task Force on Endoscopy Quality Indicators and the Murcian Health Service Task Force, especially to the co-chairs of the initiative, Fernando Carballo-Álvarez and Enrique Domínguez-Muñoz, together with the rest of collaborators: José María Bordas, Ángel Calderón, Javier Crespo-García, Jesús Espinel, José Miguel Esteban López-Jamar, Jesús García-Cano, Leopoldo López-Roses, José Miguel Marrero, Miguel Muñoz-Navas, Vicente Pons, Manuel Romero, Miguel Ángel Simón, Adolfo Suárez, Enrique Vázquez-Sequeiros; Rafael Gomis-Cebrián and José Joaquín Mira-Solves. We also thank for the non-condidionated support of SimMédica Pentax and Boston Scientific.
Conflicts of interest
The undersigned authors signed the present paper on behalf of the Sociedad Española de Patología Digestiva (SEPD). Neither the SEPD nor any of the task force members have any relationship with manufacturers of endoscopy equipment. Neither the SEPD nor any of the members of the task force have any financial interest in the companies that played a role in the research and provision of digestive endoscopy devices, albeit both the SEPD and the task force members have a sustained relationship with said companies for training, research, and improved clinical care purposes in order to promote digestive health. Finally, both the SEPD and the undersigned authors declare that the initial efforts of both local and nation-wide groups on which this review study was based were non-conditionally- supported by SimMédica Pentax and BostonScientific, who had no influence on the undertaken research, and that no third parties were involved in the discussions or development of the present paper, or had access to the contents of the final manuscript before effective publication in the Revista Española de Enfermedades Digestivas.
References
1. Donabedian A. The definition of quality and approaches to its assessment. Explorations in quality assessment and monitoring. Michigan: Health Administration Press; 1980. [ Links ]
2. America IoMUCoQoHCi. Crossing the quality chasm: A new health system for the 21st century. Washington: Press NA; 2000. [ Links ]
3. Donabedian A. Basic approaches to assessment: What to assess. Exploration, structure, process and outcomes - Quality assessment and monitoring. Vol. 1. Michigan: Health Administration Press Ann Arbor; 1980. pp. 79-122. [ Links ]
4. Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004;4(1):38. DOI: 10.1186/1472-6963-4-38. [ Links ]
5. Atkins D, Briss PA, Eccles M, et al. Systems for grading the quality of evidence and the strength of recommendations II: Pilot study of a new system. BMC Health Serv Res 2005;5(1):25. [ Links ]
6. Godolphin W. The role of risk communication in shared decision making. BMJ 2003;327(7417):692-3. DOI: 10.1136/bmj.327.7417.692. [ Links ]
7. Ley 41/2002, de 14 de noviembre, Básica Reguladora de la Autonomía del Paciente y de Derechos y Obligaciones en Materia de Información y Documentación Clínica (2002). Disponible en: https://www.boe.es/buscar/pdf/2002/BOE-A-2002-22188-consolidado.pdf. [ Links ]
8. Rizk MK, Sawhney MS, Cohen J, et al. Quality indicators common to all GI endoscopic procedures. Gastrointest Endosc 2015;81(1):3-16. [ Links ]
9. Jover R, Herráiz M, Alarcón O, et al. Clinical practice guidelines: Quality of colonoscopy in colorectal cancer screening. Endoscopy 2012;44(4):444-51. DOI: 10.1055/s-0032-1306690. [ Links ]
10. Armstrong D, Barkun A, Bridges R, et al. Canadian Association of Gastroenterology consensus guidelines on safety and quality indicators in endoscopy. Can J Gastroenterol 2012;26(1):17-31. DOI: 10.1155/2012/173739. [ Links ]
11. Gargoum FS, O'Keeffe ST. Readability and content of patient information leaflets for endoscopic procedures. Ir J Med Sci 2014;183(3):429-32. DOI: 10.1007/s11845-013-1033-8. [ Links ]
12. Calle-Urra JE, Parra-Hidalgo P, Saturno-Hernández PJ, et al. Formal quality assessment of informed consent documents in 9 hospitals. Rev Calid Asist 2013;28(4):234-43. DOI: 10.1016/j.cali.2013.01.006. [ Links ]
13. Barrio-Cantalejo IM, Simón-Lorda P, Melguizo M, et al. Validation of the INFLESZ scale to evaluate readability of texts aimed at the patient. An Sist Sanit Navar 2008;31(2):135-52. [ Links ]
14. Becker RC, Scheiman J, Dauerman HL, et al. Management of platelet-directed pharmacotherapy in patients with atherosclerotic coronary artery disease undergoing elective endoscopic gastrointestinal procedures. Am J Gastroenterol 2009;104(12):2903-17. DOI: 10.1038/ajg.2009.667. [ Links ]
15. Allen JI. Quality assurance for gastrointestinal endoscopy. Curr Opin Gastroenterol 2012;28(5):442-50. [ Links ]
16. Anderson MA, Ben-Menachem T, Gan SI, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc 2009;70(6):1060-70. [ Links ]
17. Veitch AM, Baglin TP, Gershlick AH. Guidelines for the management of anticoagulant and antiplatelet therapy in patients undergoing endoscopic procedures. Gut 2008;57(9):1322-9. [ Links ]
18. Parras FA. Clinical practice guidelines for managing coagulation in patients undergoing endoscopic procedures. Rev Esp Enferm Dig 2010;102(2):124-38. [ Links ]
19. Boustière C, Veitch A, Vanbiervliet G, et al. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2011;43(5):445-61. DOI: 10.1055/s-0030-125631720. [ Links ]
20. James PD, Antonova L, Martel M, et al. Measures of trainee performance in advanced endoscopy: A systematic review. Best Pract Res Clin Gastroenterol 2016;30(3):421-52. DOI: 10.1016/j.bpg.2016.05.003. [ Links ]
21. Dominitz JA, Ikenberry SO, Anderson MA, et al. Renewal of and proctoring for endoscopic privileges. Gastrointest Endosc 2008;67(1):10-6. DOI: 10.1016/j.gie.2007.06.020. [ Links ]
22. Anderson JT. Assessments and skills improvement for endoscopists. Best Pract Res Clin Gastroenterol 2016;30(3):453-71.23. [ Links ]
23. Burls A. AGREE II-improving the quality of clinical care. Lancet 2010;376(9747):1128-9. DOI: 10.1016/S0140-6736(10)61034-324. [ Links ]
24. Lorenzo-Zúñiga V, Moreno de Vega V, Doménech E, et al. Endoscopist experience as a risk factor for colonoscopic complications. Colorectal Dis 2010;12(10 Online):e273-7. DOI: 10.1111/j.1463-1318.2009.02146.x. [ Links ]
25. Fracchia M, Senore C, Armaroli P, et al. Assessment of the multiple components of the variability in the adenoma detection rate in sigmoidoscopy screening, and lessons for training. Endoscopy 2010;42(6):448-55. DOI: 10.1055/s-0029-1244131. [ Links ]
26. Calderwood AH, Chapman FJ, Cohen J, et al. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest Endosc 2014; 79(3):363-72. [ Links ]
27. Lieberman D, Nadel M, Smith RA, et al. Standardized colonoscopy reporting and data system: Report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc 2007;65(6):757-66. DOI: 10.1016/j.gie.2006.12.055. [ Links ]
28. Beaulieu D, Barkun AN, Dubé C, et al. Endoscopy reporting standards. Can J Gastroenterol 2013;27(5):286-92. DOI: 10.1155/2013/145894. [ Links ]
29. De Lange T, Moum BA, Tholfsen JK, et al. Standardization and quality of endoscopy text reports in ulcerative colitis. Endoscopy 2003;35(10):835-40. [ Links ]
30. Gurudu SR, Ramírez FC. Quality metrics in endoscopy. Gastroenterol Hepatol (NY) 2013;9(4):228-33. [ Links ]
31. Simón MA, Bordas JM, Campo R, et al. Consensus document of the Spanish Association of Gastroenterology on sedoanalgesia in digestive endoscopy. Gastroenterol Hepatol 2006;29(3):131-49. [ Links ]
32. Groenen MJ, Kuipers EJ, Van Berge Henegouwen GP, et al. Computerization of endoscopy reports using standard reports and text blocks. Neth J Med 2006;64(3):78-83. [ Links ]
33. Nelson DB. Infectious disease complications of GI endoscopy: Part II, exogenous infections. Gastrointest Endosc 2003;57(6):695-711. DOI: 10.1067/mge.2003.202. [ Links ]
34. Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med 1993; 118(2):117-28. [ Links ]
35. Cleaning and disinfection of equipment for gastrointestinal endoscopy. Report of a Working Party of the British Society of Gastroenterology Endoscopy Committee. Gut 1998;42(4):585-93. [ Links ]
36. Banerjee S, Shen B, Nelson DB, et al. Infection control during GI endoscopy. Gastrointestinal Endoscopy 2008;67(6):781-90. [ Links ]
37. Bordas JM, Pou Fernández JM, Nieto M, et al. Disinfection in digestive endoscopy. Current state and recommendations. Gastroenterol Hepatol 1999;22(3):157-9. [ Links ]
38. Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control. Am J Infect Control 2000;28(2):138-55. [ Links ]
39. Kruse A, Rey JF, Beilenhoff U. The European Society of Gastrointestinal Endoscopy (ESGE) - The European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) - Guidelines on Cleaning and Disinfection in GI Endoscopy - Update 1999 - Protocol for the Reprocessing of Endoscopy Accessories. Endoscopy 2000;32(1):76-83. [ Links ]
40. Rey JF. Protocol for reprocessing endoscopic accessories. European Society of Gastrointestinal Endoscopy. Endoscopy 2000;32(1):81-3. [ Links ]
41. Nelson DB, Barkun AN, Block KP, et al. Technology status evaluation report. Transmission of infection by gastrointestinal endoscopy. May 2001. Gastrointest Endosc 2001;54(6):824-8. [ Links ]
42. Rey JF, Kruse A, Neumann C, et al. ESGE/ESGENA technical note on cleaning and disinfection. Endoscopy 2003;35(10):869-77. [ Links ]
43. Rey JF, Kruse A, Endoscopy EESoG. Cleaning and disinfection in Europe according to the Endoscopic Societies' Guidelines. Endoscopy 2003;35(10):878-81. [ Links ]
44. Endoscopy ASfG. Multi-society guideline for reprocessing flexible gastrointestinal endoscopes. Gastrointest Endosc 2003;58(1):1-8. [ Links ]
45. Argaña A, Hernández-Soto E. Recomendaciones AEEED Limpieza y Desinfección en Endoscopia Gastrointestinal. Madrid: Digestiva AEEE; 2013. [ Links ]
46. Society of Gastroenterology Nurses and Associates Ic. Guideline for the use of high-level disinfectants and sterilants for reprocessing of flexible gastrointestinal endoscopes. Gastroenterol Nurs 2000; 23(4):180-7. [ Links ]
47. Society of Gastroenterology Nurses and Associates Ic. Standards of infection control in reprocessing of flexible gastrointestinal endoscopes. Gastroenterol Nurs 2000;23(4):172-9. [ Links ]
48. Santolaria S, Ducons J, Bordas JM, et al. Cleaning and disinfection in gastrointestinal endoscopy. Gastroenterol Hepatol 2007;30(1):25-35. DOI: 10.1157/13097448. [ Links ]
49. Cronmiller JR, Nelson DK, Salman G, et al. Antimicrobial efficacy of endoscopic disinfection procedures: A controlled, multifactorial investigation. Gastrointest Endosc 1999;50(2):152-8. [ Links ]
50. Foliente RL, Kovacs BJ, Aprecio RM, et al. Efficacy of high-level disinfectants for reprocessing GI endoscopes in simulated-use testing. Gastrointest Endosc 2001;53(4):456-62. DOI: 10.1067/mge.2001. 113380. [ Links ]
51. Bordas JM, Marcos-Maeso MA, Perez MJ, et al. GI flexible endoscope disinfection: "In use" test comparative study. Hepatogastroenterol 2005;52(63):800-7. [ Links ]
52. Santos SJ, Montoro M. Medidas de esterilización de endoscopios y material endoscópico accesorio. GH Continuada 2004;2(4):167-70. [ Links ]
53. González Thompson J, De la Torre Bravo A, Abdo Francis J, et al. Primer Consenso Mexicano sobre Calidad en Endoscopia Gastrointestinal. Asociación Mexicana de Endoscopia Gastrointestinal. Endoscopia 2011;23(4):195-201. [ Links ]
54. Croffie J, Carpenter S, Chuttani R, et al. ASGE Technology Status Evaluation Report: Disposable endoscopic accessories. Gastrointest Endosc 2005;62(4):477-9. DOI: 10.1016/j.gie.2005.07.005. [ Links ]
55. Alfa MJ, Sepehri S, Olson N, et al. Establishing a clinically relevant bioburden benchmark: A quality indicator for adequate reprocessing and storage of flexible gastrointestinal endoscopes. Am J Infect Control 2012;40(3):233-6. DOI: 10.1016/j.ajic.2011.02.023. [ Links ]
56. Facilities IH. Clinical Practice Parameters and Facility Standards. Ontario: Ontario CoPaSo; 2006. [ Links ]
57. Palanca I, Colomer J. Unidades asistenciales del aparato digestivo. Estándares y recomendaciones de calidad y seguridad. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad, Centro De Publicaciones; 2013. Disponible en: http://www.msssi.gob.es/organizacion/sns/planCalidadSNS/EEyRR_org.htm. [ Links ]
58. Early DS, Ben-Menachem T, Decker GA, et al. Appropriate use of GI endoscopy. Gastrointest Endosc 2012;75(6):1127-31. [ Links ]
59. Juillerat P, Peytremann-Bridevaux I, Vader JP, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Presentation of methodology, general results, and analysis of complications. Endoscopy 2009;41(3):240-6. DOI: 10.1055/s-0028-1119644. [ Links ]
60. Arditi C, Gonvers JJ, Burnand B, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Surveillance after polypectomy and after resection of colorectal cancer. Endoscopy 2009;41(3):209-17. DOI: 10.1055/s-0028-1119646. [ Links ]
61. Arditi C, Peytremann-Bridevaux I, Burnand B, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Screening for colorectal cancer. Endoscopy 2009;41(3):200-8. DOI: 10.1055/s-0028-1119644. [ Links ]
62. Schussele Filliettaz S, Gonvers JJ, Peytremann-Bridevaux I, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Functional bowel disorders: Pain, constipation and bloating. Endoscopy 2009;41(3):234-9. DOI: 10.1055/s-0028-1119644. [ Links ]
63. Peytremann-Bridevaux I, Arditi C, Froehlich F, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Iron-deficiency anemia and hematochezia. Endoscopy 2009;41(3):227-33. DOI: 10.1055/s-0028-1119644. [ Links ]
64. Schusselé Filliettaz S, Juillerat P, Burnand B, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Chronic diarrhea and known inflammatory bowel disease. Endoscopy 2009;41(3):218-26. DOI: 10.1055/s-0028-1119627. [ Links ]
65. Adler DG, Lieb JG, Cohen J, et al. Quality indicators for ERCP. Gastrointest Endosc 2015;81(1):54-66. [ Links ]
66. Ekkelenkamp VE, Koch AD, Haringsma J, et al. Quality evaluation through self-assessment: A novel method to gain insight into ERCP performance. Frontline Gastroenterol 2014;5(1):10-6. DOI: 10.1136/flgastro-2013-100334. [ Links ]
67. Costamagna G, Familiari P, Marchese M, et al. Endoscopic biliopancreatic investigations and therapy. Best Pract Res Clin Gastroenterol 2008;22(5):865-81. DOI: 10.1016/j.bpg.2008.05.004. [ Links ]
68. Froehlich F, Harris JK, Wietlisbach V, et al. Current sedation and monitoring practice for colonoscopy: An International Observational Study (EPAGE). Endoscopy 2006;38(5):461-9. DOI: 10.1055/s-2006-925368. [ Links ]
69. De Bosset V, Froehlich F, Rey JP, et al. Do explicit appropriateness criteria enhance the diagnostic yield of colonoscopy? Endoscopy 2002;34(5):360-8. DOI: 10.1055/s-2002-25277. [ Links ]
70. Bersani G, Rossi A, Ricci G, et al. Do ASGE guidelines for the appropriate use of colonoscopy enhance the probability of finding relevant pathologies in an open access service? Dig Liver Dis 2005;37(8):609-14. DOI: 10.1016/j.dld.2005.03.008. [ Links ]
71. Morini S, Hassan C, Meucci G, et al. Diagnostic yield of open access colonoscopy according to appropriateness. Gastrointest Endosc 2001;54(2):175-9. DOI: 10.1016/S0016-5107(01)70102-2. [ Links ]
72. Charles RJ, Chak A, Cooper GS, et al. Use of open access in GI endoscopy at an academic medical center. Gastrointest Endosc 1999;50(4):480-5. [ Links ]
73. López-Picazo JJ, Tomás-Garcia N, Calle-Urra JE, et al. Introduction of an accreditation system for hospital informed consent forms. Rev Calid Asist 2015;30(2):55-63. [ Links ]
74. Kopacova M, Bures J. Informed consent for digestive endoscopy. World J Gastrointest Endosc 2012;4(6):227-30. DOI: 10.4253/wjge.v4.i6.227. [ Links ]
75. Kinnersley P, Phillips K, Savage K, et al. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database Syst Rev 2013;(7): CD009445. [ Links ]
76. American Society for Gastrointestinal Endoscopy. Quality improvement of gastrointestinal endoscopy: Guidelines for clinical application. From the ASGE. Gastrointest Endosc 1999;49(6):842-4. [ Links ]
77. Faigel DO, Pike IM, Baron TH, et al. Quality indicators for gastrointestinal endoscopic procedures: an introduction. Am J Gastroenterol 2006;101(4):866-72. [ Links ]
78. Lichtenstein DR, Jagannath S, Baron TH, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc 2008;68(5):815-26. DOI: 10.1016/j.gie.2008.09.029. [ Links ]
79. Alberca-de-Las-Parras F, Marín F, Roldán-Schilling V, et al. Management of antithrombotic drugs in association with endoscopic procedures. Rev Esp Enferm Dig 2015;107(5):289-306. [ Links ]
80. Eisen GM, Baron TH, Dominitz JA, et al. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc 2002;55(7):775-9. [ Links ]
81. Zuckerman MJ, Hirota WK, Adler DG, et al. ASGE guideline: The management of low-molecular-weight heparin and nonaspirin antiplatelet agents for endoscopic procedures. Gastrointest Endosc 2005;61(2):189-94. DOI: 10.1016/S0016-5107(04)02392-2. [ Links ]
82. Alberca de Las Parras F, Egea Valenzuela J, Carballo Álvarez F. Bleeding risk in endoscopic retrograde cholangiopancreatography. Impact of the use of antithrombotic drugs. Rev Esp Enferm Dig 2017;109(3):202-10. DOI: 10.17235/reed.2017.4358/2016. [ Links ]
83. Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: Observational study. BMJ 2001;323(7323):1218-22. [ Links ]
84. Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2006;63(Suppl 4):S16-28. [ Links ]
85. Rey JF, Lambert R, Committee EQA. ESGE recommendations for quality control in gastrointestinal endoscopy: Guidelines for image documentation in upper and lower GI endoscopy. Endoscopy 2001;33(10):901-3. DOI: 10.1055/s-2001-42537. [ Links ]
86. Rex DK. Still photography versus videotaping for documentation of cecal intubation: A prospective study. Gastrointest Endosc 2000;51(4 Pt 1):451-9. DOI: 10.1016/S0016-5107(00)70447-0. [ Links ]
87. Asfeldt AM, Straume B, Paulssen EJ. Impact of observer variability on the usefulness of endoscopic images for the documentation of upper gastrointestinal endoscopy. Scand J Gastroenterol 2007;42(9):1106-12. [ Links ]
88. Non-Anesthesiologists ASoATFoSaAb. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiol 2002;96(4):1004-17. [ Links ]
89. Waring JP, Baron TH, Hirota WK, et al. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc 2003;58(3):317-22. [ Links ]
90. Barnett S, Hung A, Tsao R, et al. Capnographic monitoring of moderate sedation during low-risk screening colonoscopy does not improve safety or patient satisfaction: A prospective cohort study. Am J Gastroenterol 2016;111(3):388-94. DOI: 10.1038/ajg.2016.2. [ Links ]
91. Adler DG. Consent, common adverse events, and post-adverse event actions in endoscopy. Gastrointest Endosc Clin N Am 2015;25(1):1-8. DOI: 10.1016/j.giec.2014.09.001. [ Links ]
92. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc 2010;71(3):446-54. DOI: 10.1016/j.gie.2009.10.027. [ Links ]
93. Borgaonkar MR, Hookey L, Hollingworth R, et al. Indicators of safety compromise in gastrointestinal endoscopy. Can J Gastroenterol 2012;26(2):71-8. DOI: 10.1155/2012/782790. [ Links ]
94. Romagnuolo J, Cotton PB, Eisen G, et al. Identifying and reporting risk factors for adverse events in endoscopy. Part I: Cardiopulmonary events. Gastrointest Endosc 2011;73(3):579-85. DOI: 10.1016/j.gie.2010.11.022. [ Links ]
95. Lugay M, Otto G, Kong M, et al. Recovery time and safe discharge of endoscopy patients after conscious sedation. Gastroenterol Nurs 1996;19(6):194-200. DOI: 10.1097/00001610-199611000-00002. [ Links ]
96. Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc 2007;66(1):27-34. [ Links ]
97. Romagnuolo J, Cotton PB, Eisen G, et al. Identifying and reporting risk factors for adverse events in endoscopy. Part II: Noncardiopulmonary events. Gastrointest Endosc 2011;73(3):586-97. DOI: 10.1016/j.gie.2010.11.023. [ Links ]
98. Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med 2006;145(12):880-6. [ Links ]
99. Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol 2010;8(2):166-73. DOI: 10.1016/j.cgh.2009.10.007. [ Links ]
100. Fehmi SMA, Choksi N, Saini SD, et al. Risk of perforation during colonoscopy: A systematic review and meta-analysis. Gastroenterol 2009;136(5):A39-A. DOI: 10.1016/S0016-5085(09)60181-5. [ Links ]
101. Nelson DB, McQuaid KR, Bond JH, et al. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc 2002;55(3):307-14. [ Links ]
102. Sumanac K, Zealley I, Fox BM, et al. Minimizing postcolonoscopy abdominal pain by using CO2 insufflation: A prospective, randomized, double blind, controlled trial evaluating a new commercially available CO2 delivery system. Gastrointest Endosc 2002;56(2):190-4. DOI: 10.1016/S0016-5107(02)70176-4. [ Links ]
103. Shi H, Chen S, Swar G, et al. Carbon dioxide insufflation during endoscopic retrograde cholangiopancreatography: A review and meta-analysis. Pancreas 2013;42(7):1093-100. DOI: 10.1097/MPA.0b013e3182909da5. [ Links ]
104. Rabenstein T, Radaelli F, Zolk O. Warm water infusion colonoscopy: A review and meta-analysis. Endoscopy 2012;44(10):940-51. DOI: 10.1055/s-0032-1310157. [ Links ]
105. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth 1995;7(1):89-91. DOI: 10.1016/0952-8180(94)00001-K. [ Links ]
106. Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg 1970;49(6):924-34. DOI: 10.1213/00000539-197011000-00020. [ Links ]
107. Willey J, Vargo JJ, Connor JT, et al. Quantitative assessment of psychomotor recovery after sedation and analgesia for outpatient EGD. Gastrointest Endosc 2002;56(6):810-6. DOI: 10.1067/mge. 2002.129609. [ Links ]
108. White PF, Song D. New criteria for fast-tracking after outpatient anesthesia: A comparison with the modified Aldrete's scoring system. Anesth Analg 1999;88(5):1069-72. DOI: 10.1097/00000539-199905000-00018. [ Links ]
109. Fatima H, Rex DK. Minimizing endoscopic complications: Colonoscopic polypectomy. Gastrointest Endosc Clin N Am 2007;17(1):145-56viii. DOI: 10.1016/j.giec.2006.10.001. [ Links ]
110. Spodik M, Goldman J, Merli K, et al. Providing an endoscopy report to patients after a procedure: A low-cost intervention with high returns. Gastrointest Endosc 2008;67(1):103-11. DOI: 10.1016/j.gie.2007.08.035. [ Links ]
111. Rasool S, Ahmed S, Siddiqui S, et al. Evaluation of quality and patient satisfaction during endoscopic procedure: A cross sectional study from south Asian country. J Pak Med Assoc 2010;60(12):990-5. [ Links ]
112. Parra Hidalgo P, Bermejo Alegría RM, Más Castillo A, et al. Factors related to patient satisfaction with hospital emergency services. Gac Sanit 2012;26(2):159-65. [ Links ]
113. López-Picazo JJ, De Dios Cánovas-García J, Antúnez C, et al. Perceived quality in a dementia unit: Patients' caregivers as information providers. Neurologia 2016;pii: S0213-4853(16)30195-5. [ Links ]
114. Group JA. Global Rating Scale. Disponible en: https://www.jagaccreditation.org. [ Links ]
115. Sint Nicolaas J, De Jonge V, De Man RA, et al. The Global Rating Scale in clinical practice: A comprehensive quality assurance programme for endoscopy departments. Dig Liver Dis 2012;44(11):919-24. DOI: 10.1016/j.dld.2012.06.021. [ Links ]
116. MacIntosh D, Dubé C, Hollingworth R, et al. The endoscopy Global Rating Scale-Canada: Development and implementation of a quality improvement tool. Can J Gastroenterol 2013;27(2):74-82. DOI: 10.1155/2013/165804. [ Links ]
![]() Correspondence:
Correspondence:
Javier Júdez.
Department of Knowledge Management. SEPD.
C/ Sancho Dávila, 6.
20028 Madrid
e-mail: jjudez@sepd.es
Received: 03-05-2017
Accepted: 22-05-2017











 texto em
texto em