My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 n.7 Madrid Jul. 2017
https://dx.doi.org/10.17235/reed.2017.4569/2016
ORIGINAL PAPERS
The impact of screening on short-term outcome after surgery for colorectal cancer
Enric Sebastian1, Ricard Courtier1, Francesc Macià2, Luís Grande1,3 and Miguel Pera1,3
1Section of Colon and Rectal Surgery. Department of Surgery. Hospital del Mar. Barcelona, Spain.
2Department of Epidemiology and Evaluation. Hospital del Mar. Barcelona, Spain.
3Colorectal Cancer Research Group. Hospital del Mar-Medical Research Institute (IMIM). Barcelona, Spain
ABSTRACT
Aim: To investigate the influence of a screening program on the short-term outcome of patients undergoing surgery for colorectal cancer.
Methods: Between April 2010 and December 2012 patients diagnosed with colorectal cancer via the screening program (n = 80) were compared with patients diagnosed elsewhere (n = 106). Only patients of ≥ 50 and ≤ 69 years of age diagnosed outside the program were selected as controls. The clinical variables included age, sex, American Society of Anesthesiologists (ASA) status, Charlson index, preoperative hemoglobin and serum albumin levels, surgical approach, tumor location and stage, perioperative transfusion and postoperative morbidity. A multivariate analysis was used to identify variables independently associated with outcome.
Results: There were no significant differences with regard to age, sex and ASA status. Preoperative hemoglobin (14.1 ± 1.6 g/dl vs 12.3 ± 2.3 g/dl; p < 0.001) and serum albumin (4.45 ± 0.26 g/dl vs 4.0 ± 0.6 g/dl; p < 0.001) levels were significantly higher in the screening group. The overall morbidity was significantly lower in the screening group (38.8% vs 63.2; p < 0.001) and mainly related to a higher rate of Clavien-Dindo grade II complications in controls. There were no differences with regard to wound infection, postoperative ileus, anastomotic leakage or reoperations. The median length of hospital stay was shorter in the screening group (6 vs 9 days; p = 0.003). Multivariate analysis showed that diagnosis outside the screening program, type of surgical procedure, open surgery and Charlson index were independent risk factors for postoperative complications.
Conclusions: The diagnosis of colorectal cancer via the screening program is associated with a lower rate of postoperative minor complications and a shorter hospital stay.
Key words: Colorectal cancer. Screening. Fecal occult blood test. Surgery. Morbidity. Length of stay.
Introduction
Colorectal cancer is currently the third leading cause of cancer-related death worldwide when men and women are considered separately, and the second leading cause when both sexes are combined (1). In 2012, 32,240 new cases of colorectal cancer were diagnosed in Spain, which accounted for 15% of all cancers diagnosed. Over the same period there were 14,700 colorectal cancer-related deaths (14.7% of all cancer-related deaths), the second highest rate after lung cancer (1).
The effectiveness and the efficiency of colorectal cancer screening have been widely documented, achieving not only early cancer detection but also a reduction in mortality (2,3). In 1993, Mandel et al. (4) reported a 33% reduction of cancer-related deaths from colorectal cancer in asymptomatic patients diagnosed via a screening program based on the fecal occult blood test (FOBT) when compared with non-screened patients. An earlier tumor stage at diagnosis was also demonstrated and since then other studies have confirmed these results (5,6). A Cochrane review including these studies and their corresponding updates estimated that the implementation of colorectal cancer screening programs was associated with a reduction in mortality of 16% (7,8).
Screening has been included as a part of the recommended strategy against colorectal cancer in both the European Community Commission in 2003 and the National Spanish Health System in 2009 (9,10).
The oncological benefits of screening programs have also been demonstrated in those patients undergoing surgery with a curative intent. A recent retrospective study analyzed a cohort of 1,071 patients, 217 of which had been diagnosed via screening by colonoscopy. Patients diagnosed outside the screening program showed higher recurrence rates and a lower disease-free survival and overall survival (11).
Although the long-term benefits of colorectal cancer screening have been demonstrated extensively, the potential benefits on short-term outcome are unknown. The aim of this study was to assess whether there were differences in the short-term postoperative outcomes between colorectal cancer patients diagnosed via a screening program and those diagnosed elsewhere (outside the program).
Methods
An early detection colorectal cancer program was initiated in Barcelona in 2009 including women and men between 50 and 69 years of age, which were all residents of six districts of the city. The target population consisted of 197,839 people. The screening method used was an immunological FOBT (OC-Sensor; positivity ≥ 100 ngHb/ml buffer) test every two years. Eligible residents received an invitation letter to participate in the program and those who agreed collected and returned the test with the sample to a pharmacy within the district. Patients who tested positive were offered a colonoscopic examination. Patients diagnosed with colorectal cancer underwent staging with computerized tomography and/or magnetic resonance imaging studies and were referred for surgical treatment when appropriate.
Between April 2010 and December 2012 all consecutive patients diagnosed with colorectal cancer who underwent surgery at our institution were included in this retrospective study. Data of these patients had been prospectively collected in the hospital database. Patients who had been diagnosed within the screening program (screening group) were compared with patients who had been diagnosed elsewhere (control group). The same surgical team performed all operations. To homogenize both groups, only patients aged between ≥ 50 and ≤ 69 years of age were selected for inclusion in the control group. Patients requiring urgent surgery for bowel obstruction or perforation were excluded. Patients undergoing transanal endoscopic excision of the tumor were also excluded.
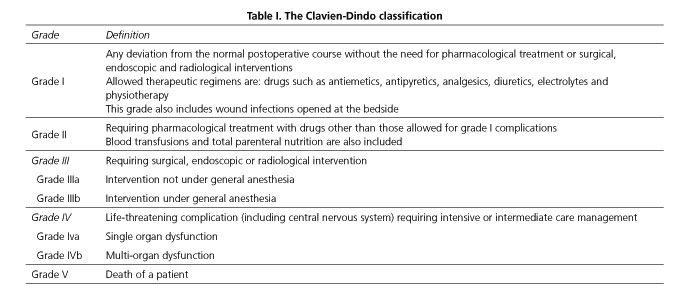
The following data were recorded in all patients: age, sex, ASA status, Charlson comorbidity index score, preoperative hemoglobin and serum albumin levels, surgical approach (open or laparoscopy), tumor location and stage (American Joint Committee on Cancer/International Union against Cancer, 7th edition), perioperative transfusion and 30-day postoperative morbidity. Hypoalbuminemia was defined when the serum albumin level was < 3.5 g/dl. The Clavien-Dindo grading system was used for the classification of postoperative complications (12) (Table I).
Postoperative ileus was considered when intolerance to diet led to a delay in discharge beyond the seventh postoperative day, the oral intake was interrupted for more than 48 hours or when insertion of a nasogastric tube was necessary.
Statistical analysis
Data from the two study groups (screening group, control group) were analyzed using the Chi-squared test or the Fisher's exact test for categorical variables and the Student's t-test or the Mann-Whitney U test for quantitative variables. Variables showing significant differences in the bivariate analysis were included in a logistic regression model to assess independent risk factors for short-term outcome after colorectal surgery. Statistical significance was set at p < 0.05. Data were analyzed using the Statistical Package for the Social Sciences (SPSS), version 20 (SPSS Inc., Chicago, IL, USA).
Results
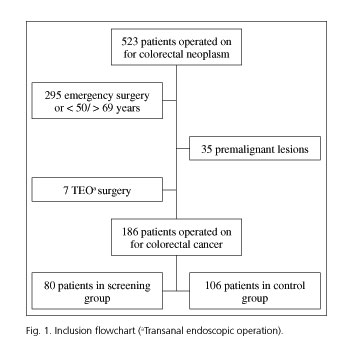
During the study period, 523 patients underwent surgery for a colorectal neoplasm (Fig. 1). A group of 80 patients were diagnosed via the screening program (screening group) and 106 patients aged between 50 and 69 years were diagnosed outside the program (controls).
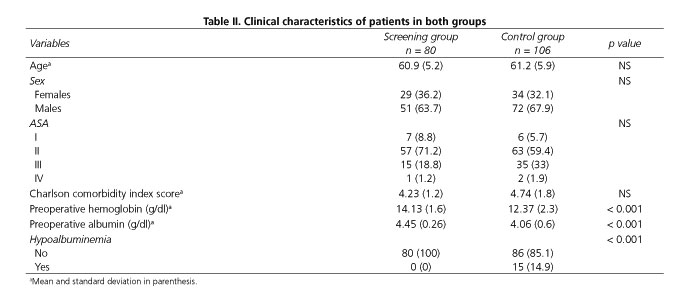
Table II shows the clinical characteristics of patients in both groups. There were no significant differences with regard to age, sex and ASA status. However, when patients were classified into ASA I and II subgroups the percentage of patients with low ASA scores was higher in the screening group (80% vs 65.1%; p = 0.026). The age-adjusted Charlson comorbidity index score was also lower in the screening group. However, the difference was not statistically significant. Preoperative hemoglobin and serum albumin levels were significantly higher in the screening group. In addition, none of the patients in the screening group presented hypoalbuminemia compared with 15% in the control group.
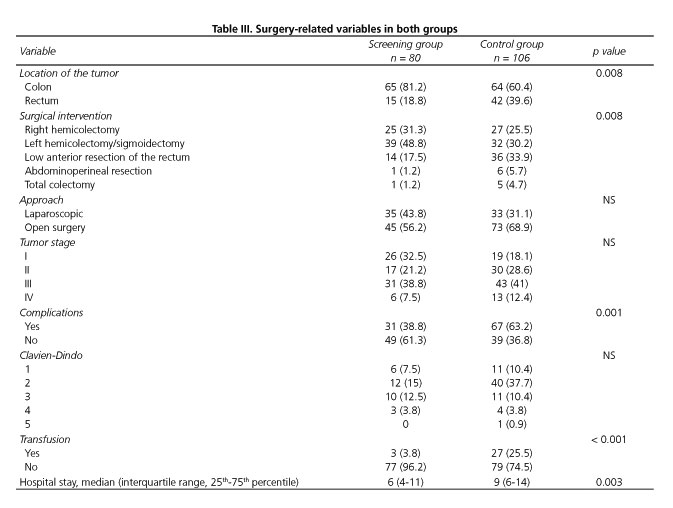
The tumor and surgery-related variables are shown in table III. The rates of patients with rectal cancer undergoing anterior resection or abdominoperineal resection were significantly higher in the control group. There were no significant differences in the surgical approach or tumor stage between groups. Postoperative morbidity was significantly lower in the screening group and the difference was mainly due to a higher rate of perioperative transfusion in controls. However, there were no differences in the rates of wound infection (10% vs 14.2%; p = 0.395), postoperative ileus (22.5% vs 23.6%; p = 0.862), anastomotic leakage (6.2% vs 4.7%; p = 0.747) or reoperations (10% vs 9.4%; p = 0.897). The length of stay was significantly shorter in the screening group. The overall morbidity in the group of patients with hypoalbuminemia was 80%.
A subgroup analysis was performed in 131 patients (66 in the screening group and 65 in the control group) after excluding patients with rectal cancer. The analysis limited to patients with colon cancer showed the same results as in the overall series. There were no differences in age (60.98 ± 5.5 vs 61.14 ± 6.2; p = 0.739), sex (women 36.4% vs 36.9%; p = 0.947), laparoscopic approach (43.1% vs 31.2%; p = 0.165), surgical procedure (p = 0.182), ASA status (p = 0.125) or Charlson comorbidity index score (4.15 vs 4.7; p = 0.067) between both groups. However, preoperative hemoglobin (14.4 vs 12.19 g/dl; p < 0.001) and serum albumin levels (4.47 vs 4.01 g/dl; p < 0.001) were significantly higher in the screening group and the percentage of patients with hypoalbuminemia was higher in the control group (0% vs 18.3%; p < 0.001). Overall postoperative complications in patients with colon cancer were also lower in the screening group (35.4% vs 62.5%; p = 0.002) as well as the rate of perioperative transfusion (3.1% vs 25%; p < 0.001). However, there were no differences in the rates of wound infection (9.2% vs 9.4%; p = 0.748), anastomotic leakage (3.1% vs 6.2%; p = 0.44) or reoperations (3.1% vs 6.2%; p = 0.44). The median (IQR) length of stay was also shorter in the screening group (6 [4-11] vs 7 [5-12] days; p = 0.026).
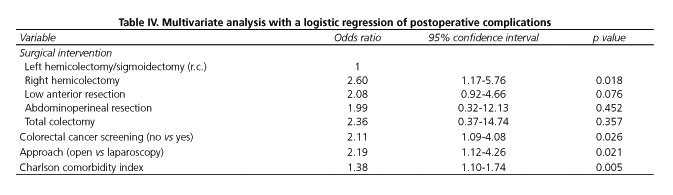
In the multivariate analysis, diagnosis outside the screening program, type of surgical procedure, open surgery and Charlson comorbidity index were independent risk factors for short-term complications (Table IV).
Discussion
This study shows that, aside from the already known benefits of screening in the long-term outcomes (increase of disease-free survival and reduction of cancer-related mortality), colorectal cancer screening is associated with better outcomes in the post-operative period (fewer complications and shorter length of hospital stay).
The difference in postoperative morbidity was mainly related to a higher percentage of grade II complications of the Clavien-Dindo classification in the group of patients diagnosed elsewhere and, in particular, to a greater need for perioperative transfusion. In part, this is because the patients in the control group had lower preoperative hemoglobin levels. Other factors such as a greater number of stage I patients and a higher rate of a laparoscopic approach in the screening group (although not statistical significant) could have had an impact on the reduced need for transfusion. On the other hand, perioperative transfusion is an independent risk factor for a worse oncological outcome (13,14).
The nutritional status is a key factor in cancer patients undergoing surgery (15). The serum albumin is a simple estimation of the visceral protein and is one of the best parameters to assess the nutritional condition. Several studies have found an association between serum albumin concentration and in-hospital mortality (16-18), postoperative complications (18,19), duration of hospitalization (18,20), quality of life (21) and prognosis in cancer patients (22-25). Patients from the screening program had a better nutritional status based on preoperative serum albumin levels. It is important to note that 15% of patients in the control group presented hypoalbuminemia compared with zero patients in the screening group. Even though the control group showed a greater overall morbidity, no differences were found regarding major postoperative complications such as anastomotic leakage or the need for reoperation. It is worth noting that the mean serum albumin level in both groups was above 4 g/dl, and in most of the studies conducted to assess the relationship between serum albumin and morbidity the cut-off for serum albumin was established at < 3.5 g/dl. This could explain why the statistically significant differences in serum albumin levels had no major clinical implications. On the other hand, lower levels of preoperative serum albumin have been associated with a higher cancer-related mortality and a lower overall survival in patients with colorectal cancer and other malignancies (15,17,22-25). Therefore, the differences in serum albumin may have long-term implications which are not reflected in this study.
Another finding of the present study related with short-term postoperative outcomes is the assessment of the surgical risk. The percentage of patients with low ASA I-II scores was greater in the screening group. Moreover, the age-adjusted Charlson comorbidity index score was lower in the screening group. Although the difference did not reach statistical significance. In this regard, several studies have demonstrated that both the ASA status and the comorbidities are associated with early postoperative outcomes (19,26-29).
One of the limitations of the present study is the higher percentage of patients with rectal cancer in the control group, which could create a bias and have a direct influence on the results (19,28). For this reason, an analysis that excluded patients undergoing low anterior resection or abdominoperineal excision of the rectum was conducted, and statistically significant differences in the preoperative hemoglobin and albumin levels, need for transfusion, overall morbidity and hospital stay were also found. With regard to major postoperative complications, there were no differences in paralytic ileus, anastomotic leakage or reoperation. In the multivariate analysis, surgery for rectal cancer was not an independent factor for the occurrence of complications.
Further studies with larger cohorts are needed to demonstrate whether screening programs are also associated with a reduction of major complications in patients undergoing colorectal cancer surgery.
Conclusions
In this retrospective cohort of patients, diagnosis of colorectal cancer via the screening program was associated with a lower rate of minor complications and a shorter hospital stay. These differences maintained when only patients with colon cancer were analyzed. The decrease in morbidity was mainly due to a lower percentage of grade II Clavien-Dindo complications.
Acknowledgements
The authors would like to thank Marta Pulido, MD, PhD, a freelance editor for editing the manuscript and editorial assistance, and Sergi Mojal, BsC, for statistical review of the study.
References
1. Globocan 2012. Cited 2015 Mar 28. Available at: http://globocan.iarc.fr/Default.aspx. [ Links ]
2. Roxburgh CSD, McTaggart F, Balsitis M, et al. Impact of the bowel-screening programme on the diagnosis of colorectal cancer in Ayrshire and Arran. Colorectal Dis 2013;15(1):34-41. DOI: 10.1111/j.1463-1318.2012.03100.x. [ Links ]
3. Mackay C, Ramsay G, Rafferty A, et al. Impact of the Scottish Bowel Cancer Screening Programme on patient and tumour characteristics at a single centre. J Eval Clin Pract 2014;20(1):7-11. DOI: 10.1111/jep.12071. [ Links ]
4. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993;328(19):1365-71. DOI: 10.1056/NEJM199305133281901. [ Links ]
5. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348(9040):1472-7. DOI: 10.1016/S0140-6736(96)03386-7. [ Links ]
6. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348(9040):1467-71. DOI: 10.1016/S0140-6736(96)03430-7. [ Links ]
7. Towler BP, Irwig L, Glasziou P, et al. Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane database Syst Rev 2000;(2):CD001216. [ Links ]
8. Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am J Gastroenterol 2008;103(6):1541-9. DOI: 10.1111/j.1572-0241.2008.01875.x. [ Links ]
9. Estrategia en cáncer del Sistema Nacional de Salud. Madrid: Ministerio de Sanidad y Consumo; 2006. pp. 279. Cited 2015 Mar 28. Available at: http://www.msssi.gob.es/eu/organizacion/sns/planCalidadSNS/pdf/excelencia/cancer-cardiopatia/CANCER/opsc_est1.pdf.pdf. [ Links ]
10. Estrategia en Cáncer del Sistema Nacional de Salud Actualización aprobada por el Consejo Interterritorial del Sistema Nacional de Salud, el 22 de octubre de 2009. Cited 2015 Mar 28. Available at: http://www.msssi.gob.es/organizacion/sns/planCalidadSNS/pdf/ActualizacionEstrategiaCancer.pdf. [ Links ]
11. Amri R, Bordeianou LG, Sylla P, et al. Impact of screening colonoscopy on outcomes in colon cancer surgery. JAMA Surg 2013;148(8):747-54. DOI: 10.1001/jamasurg.2013.8. [ Links ]
12. Clavien PA, Barkun J, De Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg 2009;250(2):187-96. DOI: 10.1097/SLA.0b013e3181b13ca2. [ Links ]
13. Gunka I, Dostalik J, Martinek L, et al. Impact of blood transfusions on survival and recurrence in colorectal cancer surgery. Indian J Surg 2013;75(2):94-101. [ Links ]
14. Schiergens TS, Rentsch M, Kasparek MS, et al. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum 2015;58(1):74-82. [ Links ]
15. Kuzu MA, Terzio lu H, Genç V, et al. Preoperative nutritional risk assessment in predicting postoperative outcome in patients undergoing major surgery. World J Surg 2006;30(3):378-90. [ Links ]
16. Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg 1999;134(1):36-42. [ Links ]
17. Longo WE, Virgo KS, Johnson FE, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum 2000;43(1):83-91. [ Links ]
18. Kudsk KA, Tolley EA, DeWitt RC, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr 2003;27(1):1-9. [ Links ]
19. McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015;102(5):462-79. [ Links ]
20. Chima CS, Barco K, Dewitt MLA, et al. Relationship of Nutritional Status to Length of Stay, Hospital Costs, and Discharge Status of Patients Hospitalized in the Medicine Service. J Am Diet Assoc 1997;97(9):975-8. [ Links ]
21. Lis CG, Gupta D, Lammersfeld CA, et al. Role of nutritional status in predicting quality of life outcomes in cancer - a systematic review of the epidemiological literature. Nutr J 2012;11:27. [ Links ]
22. Sun L-C, Chu K-S, Cheng S-C, et al. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer 2009;9:288. [ Links ]
23. Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol 2006;40(7):592-5. [ Links ]
24. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J 2010;9(1):69. [ Links ]
25. Ishizuka M, Nagata H, Takagi K, et al. Inflammation-Based Prognostic Score Is a Novel Predictor of Postoperative Outcome in Patients With Colorectal Cancer. Ann Surg 2007;246(6):1047-51. [ Links ]
26. Ostenfeld EB, Nørgaard M, Thomsen RW, et al. Comorbidity and survival of Danish patients with colon and rectal cancer from 2000-2011: a population-based cohort study. Clin Epidemiol 2013;5(Suppl 1):65-74. [ Links ]
27. Alves A, Panis Y, Trancar D. Factors associated with clinically significant anastomotic leakage after large bowel resection: Multivariate analysis of 707 patients. World J Surg 2002;26(4):499-502. [ Links ]
28. Fazio VW, Tekkis PP, Remzi F, et al. Assessment of operative risk in colorectal cancer surgery: The Cleveland Clinic Foundation Colorectal Cancer Model. Dis Colon Rectum 2004;47(12):2015-24. [ Links ]
29. Lemmens VEPP, Janssen-Heijnen MLG, Verheij CDGW, et al. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg 2005;92(5):615-23. [ Links ]
![]() Correspondence:
Correspondence:
Enric Sebastian Valverde.
Section of Colon and Rectal Surgery.
Department of Surgery.
Hospital del Mar.
Passeig Marítim, 25-29.
08003 Barcelona, Spain
e-mail: enricsebas@hotmail.com
Received: 26-08-2016
Accepted: 14-03-2017