Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.7 Madrid jul. 2017
https://dx.doi.org/10.17235/reed.2017.4065/2015
REVIEW
Serrated lesions and serrated polyposis syndrome
Lesiones serradas y síndrome de poliposis serrada
Alberto Herreros-de-Tejada1, Carmen González-Lois2 and José Santiago1
Services of 1Digestive Diseases and 2Pathology. IDIPHIM. Hospital Universitario Puerta de Hierro Majadahonda. Madrid, Spain
ABSTRACT
The serrated pathway has been shown to be an alternative colorectal carcinogenetic route potentially accounting for up to one third of all CRCs. Serrated lesions, particularly SSPs, have been a focus of research during the past few years. They have well-established histological and molecular characteristics that account for their potential carcinogenetic risk through the accumulation BRAF, KRAS and methylator profile (CpG) mutations. Their endoscopic identification and resection represent a challenge because of their specific characteristics, and the need for an adequate specimen for histological diagnosis. Knowledge of these lesions is key, as is the adoption of established criteria for their endoscopic description and histological diagnosis. SPS is the maximum expression of involvement by serrated lesions, is associated with increased risk for CRC, and requires attentive endoscopic follow-up, as well as family screening. While the exact etiopathogenic mechanism remains unknown, current research will likely provide us with appropriate answers in the not too distant future.
Key words: Colonic polyps. Colorectal cancer. Serrated polyposis syndrome. Serrated polyposis. Hyperplastic polyposis syndrome. Hyperplastic polyps. Sessile serrated polyps. Serrated pathway.
RESUMEN
La vía serrada se ha demostrado como vía alternativa de carcinogénesis colorrectal que podría explicar hasta un tercio de todos los CCR. Las lesiones serradas, en particular los PSS, han sido objeto de estudio en los últimos años. Presentan características histológicas y moleculares definidas, que explican su potencial riesgo de carcinogénesis mediante acúmulo de mutaciones BRAF, KRAS y perfil metilador (CgP). Su detección y resección endoscópica plantean un desafío por sus particulares características, así como por la necesidad de contar con un adecuado espécimen para su diagnóstico histológico. Es esencial el conocimiento de estas lesiones, así como la adopción de criterios definidos para su descripción endoscópica y diagnóstico histológico. El SPS es la expresión máxima de afectación por lesiones serradas, se asocia con un riesgo aumentado de CCR y requiere un estrecho seguimiento endoscópico, así como un cribado familiar. Aunque aún no se ha podido establecer el mecanismo etiopatogénico exacto, es probable que las vías de investigación en marcha puedan darnos una respuesta en un futuro no muy lejano.
Palabras clave: Pólipos colónicos. Cáncer colorrectal. Síndrome de poliposis serrada. Poliposis serrada. Síndrome de poliposis hiperplásica. Pólipos hiperplásicos. Pólipos serrados sesiles. Vía serrada.
Introduction
Serrated lesions result from alternative carcinogenesis in colorectal cancer (CRC), the so-called "serrated pathway" (1). These lesions have been the subject of study and attention in the last few years, and are considered to account for up to one third of all CRCs (2-7). Failure to detect these lesions may possibly result in a significant number of interval neoplasms for different reasons (challenging identification, predominance in the right colon, incomplete resection from poorly identified lesion margins, etc.) (5,8-11), and indirectly cause a lower effectiveness of colonoscopy in the prevention of proximal CRC (12-14).
Serrated pathway carcinogenesis: epidemiology and pathophysiological, molecular and genetic characteristics
The "serrated" carcinogenesis pathway, which differs from the "traditional" one and from the precursor "serrated lesions", accounts for up to 20-30% of all CRCs (4-7). The initial description of adenocarcinomas arising from serrated lesions was reported by Jass and Smith in 1992 (15), with tobacco exposure defined as a risk factor and no conclusive association with ethnic factors (7). The same carcinogenetic pathway would result in adenocarcinomas with varying biological behavior, histology and molecular profiles.
In the serrated pathway, signals are activated within the mitogen-activated protein kinase (MAPK) pathway, also known as the RAS-RAF-MAPK cascade, with predominant BRAF or KRAS mutations and CpG island methylator phenotype (CIMP), both high-level (CIMP-H) and low level (CIMP-L). The molecular study of adenocarcinomas related to serrated lesions also reveals a smaller proportion of CRCs with no BRAF or KRAS mutations. Approximately 30% of CRCs evolve through the serrated pathway, 10% have BRAF mutations, 15% have KRAS mutations, and 5% have both (16). BRAF-mutated CRCs are found proximally, are CIMP-H, and may exhibit microsatellite instability (MSI) o microsatellite stability (MSS). The sessile serrated polyp (SSP) is considered as their precursor lesion, from which dysplasia subsequently develops. KRAS-mutated CRCs are located preferentially in the distal colon, with CIMP-L and MSS. Their precursor lesion is thought to be the traditional serrated adenoma (TSA). Serrated-pathway CRCs are usually mucosecretory and express MUC2, MUC5B, MUC5A, and MUC6. Other typical histological features, most commonly in CRCs with MSI, include poor differentiation, intratumoral lymphocytosis, and a peritumoral Crohn-like inflammatory response (16).
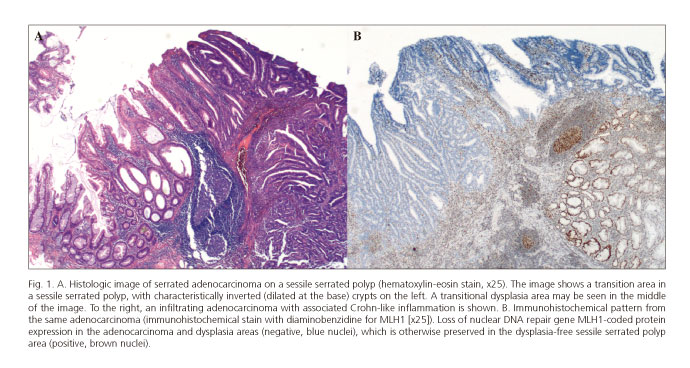
Histological subtype definitions have been described for this pathway (serrated adenocarcinoma, medullary carcinoma); however, despite their recognition as variants in the WHO classification (17), no direct correspondence with specific molecular profiles can be found. Hence, the sole histological feature useful for the diagnosis of serrated-pathway CRC is its continuity with a precursor lesion (Fig. 1A).
Serrated lesions: histologic types and endoscopic characteristics
Serrated lesions represent approximately one third of all colonic polyps (18,19), with a prevalence of 20-35% among the general population (20,21). Serrated lesions show hystopathological features unlike conventional adenomas, and endoscopic appearance partly correlate to histological subtype (22). The term "serrated adenoma" was first used by Longacre and Fenoglio-Preiser in 1990 (23) to describe lesions sharing hyperplastic polyp (HP) and adenoma features. Later, Torlakovic and Snover used the term "sessile serrated adenoma" in the setting of hyperplastic polyposis syndrome (24). The "sawtoothed" appearance of the crypt epithelium is the most typical morphologic feature. It results from expansion of the mid- or upper-crypt proliferative area in association with inhibited cell exfoliation and inhibited apoptosis (25).
Histologic diagnosis of serrated lesions is challenging, with low inter-observer concordance, which has been classically attributed to two major issues:
- The histological differential diagnosis between hyperplastic polyp (HP) and SSP without dysplasia is challenging in endoscopic biopsy samples, limited by deficient orientation or fragmentation.
- Different guidelines provide different terminologies and minimal diagnostic criteria (6,13,17).
A major goal in order to advance in the study of these lesions is a homogeneous terminology and clearly defined minimal diagnostic criteria. In this regard, recently reported diagnostic guidelines recommend a simplified classification system (6):
1. Hyperplastic polyp (HP).
2. Sessile serrated polyp (SSP) (without/with dysplasia).
3. Traditional serrated adenoma (TSA).
4. Mixed polyp: this category, still unrecognized by the WHO classification, is included in these guidelines for lesions with HP-like diagnostic characteristics and "classic" adenoma features in the left colon. When described in the right colon, most lesions are SSP with dysplasia. In any case, the diagnosis of these lesions is controversial, with no complete consensus in the literature.
In this review we primarily consider the lesions recognized by the WHO, which are discussed in detail below.
Hyperplastic polyp
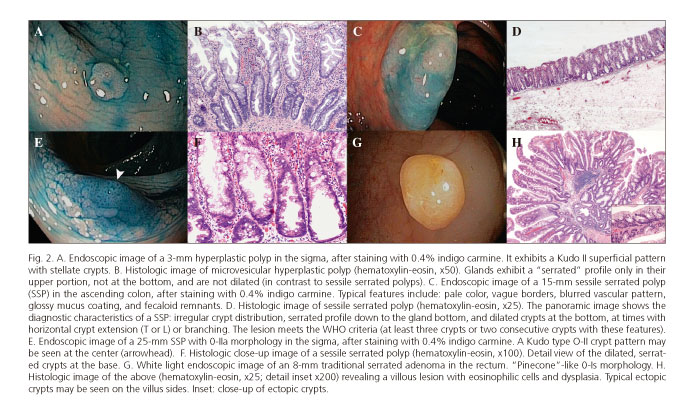
These lesions make up the most numerous group of serrated lesions (85%) (26,27). They are about 5 mm in size, pale or mucosa-like in color, and most are located in the sigma and rectum (6,22). Their mucosal pattern with star-shaped crypts (Kudo II pattern with magnification endoscopy) is characteristic (Fig. 2A). Histologically, glands exhibit a saw-toothed profile without dysplasia or histologic features, preventing them being categorized as SSPs (17).
Three morphological patterns exist:
1. Microvesicular HP: mucosecretory epithelial cells and reduced goblet-cell density (Fig. 2B). They usually have BRAF mutations.
2. HP rich in goblet cells: usually associated with KRAS mutations.
3. HP poor in mucin: scarce, similar to microvesicular, less mucin.
High grade dysplasia in HP is exceptional, and the risk for CRC is negligible for smaller lesions in the left colon (6).
Sessile serrated polyp (SSP)
These lesions are a minor subgroup among serrated lesions, with an overall prevalence of 2-13% (5,11,18,19,26-31); however, they are considered to be potentially neoplastic, with a prevalence of high-grade dysplasia at 10-13% (22,23,29), particularly when larger than 10 mm (32).
They are characterized by sessile or a flat morphology (0-Is /0-II), pale color similar to the normal mucosa, fuzzy vascular pattern, and poorly delineated limits, and are usually covered by a yellowish mucus layer that may hide them from sight in the presence of adhered fecaloid remnants (13,26,33,34) (Fig. 2C). One third of cases may exhibit an erythematous surface, and the mean size is above 10 mm (22,33). Most (> 80%) are located proximally to the descending colon (34). Their superficial crypt pattern may be similar to that of HPs, but a Kudo II open shape (II-O) has been specifically described, with larger oval crypts (Fig. 2E). This pattern is highly predictive of SSP, with a specificity of 97.3% (34,35) and limited sensitivity at < 66% (36). However, the II-O pattern seems to be a marker for the CpG methylator phenotype and microsatellite instability, which would define a premalignant status (35). Another interesting characteristic that has been studied with NBI + magnification is the presence of "varicose" vasculature (varicose microvascular vessels), defined as tortuous vascular structures larger than mucosal vessels in diameter, which seemingly are highly specific (88%) (33).
In the literature the terms sessile serrated adenoma and sessile serrated polyp are often used interchangeably. The WHO has attempted to unify both terms with the designation "sessile serrated adenoma/polyp" (SSA/P) (6,17). However, the recently reported British guidelines on serrated lesions recommend that only SSP be used (6), since the term adenoma implies the presence of dysplasia, which does not occur in all SSPs. The morphological features distinguishing them from HPs are listed in figures 2D and F.
The current guidelines differ regarding minimal diagnostic criteria:
- The WHO establishes a minimum of three crypts, or two adjacent crypts with the typical morphological features, in any single lesion (17).
- The American Gastroenterology Society recommends only one crypt with one or more characteristic features (13).
Histological and endoscopic differentiation between HP and SSP depends on a variable agreement rate and is a controversial topic in daily clinical practice (37). A recent study with NBI and magnification showed a high diagnostic yield for three combined characteristics (varicose vascular pattern, size > 10 mm, and location in the right colon) when distinguishing SSPs from HPs larger than 6 mm (33). Despite efforts to establish diagnostic criteria in the past few years, both the endoscopist and pathologist must have a high suspicion index, particularly for SSP (38). According to the WHO criteria, a continuous spectrum persists between microvesicular HP and SSP. SSPs are usually larger than 10 mm, and almost certainly will meet such minimal criteria, preferably in complete, well-oriented, non-fragmented resections. There are indeterminate lesions that do not meet WHO minimal criteria, usually as they are smaller, but do contain some crypts with characteristic features. Diagnostic correlation increases when lesion location and size is known (39).
SSP identification during endoscopy is particularly challenging, and detection rates among endoscopists (23-65%) (19,28,30,40) are highly variable, much more so than for adenomas (19), which likely indicates that a significant number of lesions remain undiagnosed in clinical practice (13). An experienced endoscopist may identify an SSP in the right colon in up to 20% of procedures (31,40). Therefore, such lesions must be proactively searched for with an adequate withdrawal time and systematic, careful examination (40). The challenging nature of endoscopic SSP diagnosis, and its likely relationship with interval CRC, have led some authors to suggest a 5-10% minimal detection rate for these lesions as quality criterion with a high discriminating power in the mid-risk population (28,31,36,40,41), although no evidence similar to that supporting the role of adenoma detection rates in reducing CRC incidence and CRC-related mortality is available yet (42,43).
SSP with dysplasia
This is a well-established class among SSPs. Dysplasia is defined as the morphologic aspect of intraepithelial neoplasm in the colon and rectum, histologically recognized in classic adenomas. Dysplasia, according to the WHO classification, may be high-grade or low-grade (6,17). In this context, dysplasia is commonly associated with the absence of the DNA repair protein hMLH-1 within the carcinogenic pathway including BRAF mutations and diffuse DNA methylation (CIMP-H) (Fig. 1B). In comparison with adenomas, dysplasia is also more common in lesions > 10 mm (29). Data remain inconclusive on SSP-associated risk for CRC. It is mostly accepted that larger lesions with dysplasia represent the highest risk for progression (6).
Traditional serrated adenoma (TSA)
These are uncommon lesions with an estimated prevalence < 1% among the general population (18,27). Their morphology is often protusive (0-Ip) and exophytic, with a mean size greater than 10 mm, the surface color is erythematous, and they are distributed in the entire colon but they are most commonly distal (22,41,44,45). Their typical macroscopic shape is referred to as "pinecone"-like (Fig. 2G) or double elevation (22,46), although shapes similar to those of classic adenomas are also common (47). Under magnification, characteristic crypt Kudo patterns include the IIIL "stellar", III-H "fern-like" (22,46), and IV-S "saw-toothed" types (34,35), but their actual diagnostic yield has been questioned. Given the nature of these lesions, some cases exhibit a melting pot appearance including several patterns (II, IIIs, IIIL) (47).
The incidence of high-grade dysplasia is lower than 2% (22). Fifty per cent of cases are said to be associated with a HP or SSP component in the same lesion, or with a tubular or tubulovillous adenoma in their vicinity (44,45). The association with HP or SSP within the same lesion correlates with a colonic location, similar to the way these two lesions present in clinical practice (48).
Characteristically, cells have an eosinophilic cytoplasm with long nuclei (Fig. 2H). Their serrated appearance results from a combination of undulated crypt epithelium and crypt budding (ectopic crypts). Sessile serrated polyps have no budding. They are characterized by disrupted cell control and programmed cell death signaling pathways, which results in enhanced cell proliferation. Such increased cell proliferation leads to the accumulation of somatic mutations, which may account for the faster development of traditional serrated adenomas when compared to conventional adenomas (6).
Miscellanea: special situations where serration may develop
Serrated glands have been shown to develop in chronic inflammatory bowel disease, with or without dysplasia, and in association with stromal lesions (neurofibromas, perineuromas, fibroblastic polyps, submucosal lipomas) (6). Whether this is due simply to comorbidity or secondary serration has not be determined.
General recommendations from a histological viewpoint
The desirable goal in the diagnosis of serrated lesions is increased histological recognition and inter-observer agreement, which requires:
- Differentiating SSPs and HPs by applying the criteria established in diagnostic guidelines.
- Obtaining a fine lesion en-bloc resection (preferable to endoscopic biopsy) for improved orientation and assessment of completeness.
- Obtaining a good correlation with endoscopic data to assess lesion risk, particularly regarding size and location.
- Using the nomenclature recommended by guidelines (WHO) to increase consistency and improve data collection for the study of these lesions.
- Assessing dysplasia in this context according to the already established dysplasia criteria for "classic" adenomas.
Serrated polyposis syndrome (SPS)
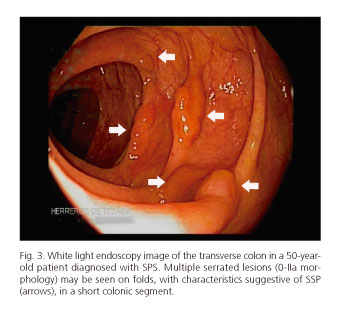
Serrated polyposis syndrome (SPS) is characterized by multiple serrated lesions (SSP/HP) (Fig. 3), and defined by one or more than three WHO-defined criteria (17,25):
1. Presence of at least five serrated lesions proximal to the sigma, with at least two of them larger than 10 mm.
2. Any number of serrated lesions proximal to the sigma in an individual with a first-degree relative diagnosed with SPS.
3. Over 20 serrated lesions spread along the whole colon, regardless of size.
Clinically, three disease phenotypes may be distinguished, which might even translate to different etiopathogenic substrates. Phenotype 1 represents the most common subtype (50% of cases) and is characterized by a reduced number of large serrated polyps in the right colon. In contrast, phenotype 2 is characterized by a high number of smaller serrated lesions in the left colon. Lastly, phenotype 3 (30% of cases) represents a mixture of the other two phenotypes (49).
Epidemiology and risk factors
The prevalence of SPS is poorly understood, but a greater awareness of endoscopists and pathologists of these conditions, together with diagnostic advancements, has clearly increased the recognition of serrated lesions and, consequently, SPS (50). Overall, its prevalence seems lower than 0.09% in colonoscopy-based CRC screening programs (51), but is considerably higher in preselected (positive fecal occult blood test) populations, with estimates of 0.34-0.66% (52,53). SPS prevalence is higher than for other polyposis syndromes, including familial adenomatous polyposis (54). The mean age at diagnosis is 55 years, with no differences between genders (49,55,56). Clear demographic, histopathologic, or molecular differences do not exist between phenotypes, and a greater proportion was only shown among first-degree relatives with CRC for phenotype 2 (numerous smaller lesions mainly in the left colon) (57). Several studies have associated smoking and being overweight with the development of serrated lesions and SPS (56,58,59).
Diagnosis and etiopathogenesis
This is a highly heterogeneous clinical condition with a poorly understood etiopathogenesis and natural history. While most SPS cases are sporadic, evidence suggests that this syndrome exhibits a genetic component at least occasionally. The higher prevalence of CRC and SPS in first-degree relatives (FDRs) as compared to the general population supports this theory (49,55,56,60-68), although the genetic basis and its heritance pattern is not fully understood yet (60,66). The action of various environmental factors on an unknown genetic predisposition will likely condition specific disease expression (49).
The exclusive use of clinical diagnostic criteria, which may somehow be considered to be both arbitrary and restrictive, largely results from unfamiliarity with the condition itself. Thus, difficulties in the endoscopic recognition of serrated lesions, together with the challenges often entailed by their correct histological characterization, commonly results in under-diagnosis.
Molecular characteristics of serrated polyps in SPS
The prevalence of KRAS and BRAF mutations in serrated polyps is 64-75% (66,69). Those mutations are more common in serrated polyps from patients with SPS than in sporadic serrated polyps, hence they could be used as a criterion supporting the diagnosis of SPS (57,66). Interestingly, dysplasia develops more commonly in lesions with BRAF and KRAS mutations (69). In fact, almost 90% of CRCs with the CpG island methylator phenotype (CIMP+) characteristic of the serrated pathway have BRAF and/or KRAS mutations (70). This fact may indicate a higher risk for the development of dysplasia foci in serrated polyps in the SPS setting.
Increased risk for CRC
The association of SPS and increased CRC prevalence/incidence (25-70%) is a classic description (55,60,68,71,72). Recent studies seem to reduce the estimated risk by about 15% (56), with a cumulative risk for interval cancer at five years of 2-7% (8,56,65) regardless of the phenotype (56,57). The presence of at least two SSPs proximal to the splenic angle, or of high-grade dysplasia in any SSP, seems to be a factor associated with increased risk for CRC development (56).
SPS and first-degree relatives
It has been shown that over 30% of first-degree relatives of patients meeting SPS diagnostic criteria are themselves diagnosed with SPS during screening colonoscopy (55,56,60-64), mainly using the WHO's second criterion (64). An association of CRC has been reported for up to 10-50% of FDRs, and the risk for CRC in FDRs is estimated to be 5-fold that of the general population (49,55,65-68). In contrast to other hereditary syndromes, evidence for an increased incidence of extracolonic neoplasms in patients with SPS or their FDRs has been insufficiently proven (49,73).
Endoscopic follow-up and screening for families
Current clinical guidelines recommend that these patients be monitored annually with colonoscopy, attempting to resect at least all serrated lesions > 3-5 mm, with special emphasis in the right colon (13). However, recent studies seem to suggest a subgroup of patients with a lower risk profile (absence of SSPs proximal to the splenic angle, with high-grade dysplasia) where monitoring could be performed less frequently than 3-year intervals (56). In cases not amenable to endoscopic control because of the number or characteristics of serrated lesions, or when a CRC is identified, a surgical approach is recommended (subtotal/total colectomy) (13).
It is currently recommended that all first-degree relatives undergo screening colonoscopy starting at 35-40 years of age, or ten years earlier than the age of SPS diagnosis when diagnosed at a younger age. Recommended subsequent monitoring should be at 5-year intervals, except when lesions are present (13,55,64).
Endoscopic technical aspects for the identification of serrated lesions
Withdrawal time
As seen for adenoma identification (74), withdrawal time is key for the identification of serrated lesions, particularly of SSPs (28,40). Recently, an important study highlighted the relevance of lengthening withdrawal time to nine minutes in order to obtain 30% higher recognition rates for serrated lesions (SSPs and HPs) as compared to six minutes (28).
Preparation
Although numerous studies have shown that adequate preparation correlates to higher adenoma detection rates (75,76), this relationship could not be specifically and unequivocally established for serrated lesions (40,77). However, indirect evidence suggests that suboptimal preparation is associated with lower detection rates for non-polypoid lesions (78), among which serrated lesions may be reasonably expected.
Indigo carmine chromoendoscopy
Indigo carmine panchromoendoscopy has indirectly proven effective for detecting serrated lesions in a number of studies (79,81). However, its usefulness has not been shown consistently (47,82), and SPS is likely the sole scenario where it may be recommended for routine use until further studies are available (82).
Narrow band imaging (NBI) chromoendoscopy
The application of the characteristics included in the NICE (NBI International Colorectal Endoscopic Classification) (83) has been specifically studied for the characterization of SSP-like serrated lesions starting with four endoscopic features: cloud-like surface, borders indistinguishable from the normal mucosa, surface dark spots, and irregular borders (50). The promising results initially obtained in the ex vivo study (50) could not be replicated in clinical practice (84), hence no definitive conclusions may be drawn.
Endoscopic management and follow-up for serrated lesions
As a general recommendation, serrated lesions smaller than 5 mm, with hyperplastic appearance, located in the sigma and rectum should not be systematically resected (13,41) given that their malignant potential is extremely rare, and are considered as a normal finding by post-colonoscopy follow-up guidelines (85). Real-time endoscopic assessment with NBI has proven a high-yield strategy for the differentiation of HPs from adenomatous lesions (86). Some authors recommend endoscopic resection for all serrated lesions that are proximal to the sigma, particularly those with endoscopic characteristics suggestive of SSP or TSA (13,41). However, the resection of lesions > 3 mm will likely be insufficient to provide a protective effect of colonoscopy for CRC (87).
As a general approach, virtually the same principles regarding polypectomy and endoscopic mucosal resection (EMR) for adenomatous lesions apply to serrated lesions, with the following peculiarities. Complete, en-bloc resection should be attempted for serrated lesions whenever possible (88). When unfeasible, residual tissue at resection margins should be excised with biopsy forceps or treated with argon plasma (47). Flat morphology (0-II), poorly defined borders, extension to colonic folds, and preferential location at the proximal colon (26,33,34) are probably factors leading to significant rates of incomplete resection as seen in some studies for serrated lesions > 10 mm (> 30%) (89), which makes endoscopic follow-up at 3-6 months advisable according to some authors (38). Virtual chromoendoscopy (NBI), either superficial or submucosal, must be attempted with the indigo carmine stain to aid border definition prior to EMR (13,38,47), emphasizing the importance of safe surgical margins. Furthermore, serrated lesions are usually soft, slippery growths commonly seen on folds, which require a stiff or monofilament snares to firmly grab the lesion and adjacent normal mucosa (38,41).
All endoscopists should not overlook the importance of specimen preparation to facilitate its pathological study. Extension and fixation on a flat surface, be it blotting paper or a harder material (rubber, cork) for larger lesions, is strongly recommended (90,91). Adequate serrated lesion preparation and extension has been shown to significantly increase the SSP-related diagnostic rate and inter-pathologist agreement (90).
Resection with biopsy forceps (1-3 mm) or the cold snare technique (> 3 mm) is acceptable for smaller lesions (< 6-8 mm) (13,92,93). The technique of choice for larger lesions is hot-snare EMR (47). Various studies have specifically addressed hot-snare EMR for SSPs > 20 mm, with resection rates > 95% and complication rates < 15% (94). Nonetheless, late recurrence is a limitation of fragmented EMR for these lesions, with rates approaching 20% (95). More extensive studies including adenomatous and serrated lesions > 20-40 mm using fragmented EMR have shown success rates above 90%, albeit with a persisting relapse risk, particularly for lesions > 40 mm (96,97). A recently used technique for large serrated lesions is endoscopic submucosal dissection, which allows en-bloc resection with no size limitations for colorectal lesions (98).
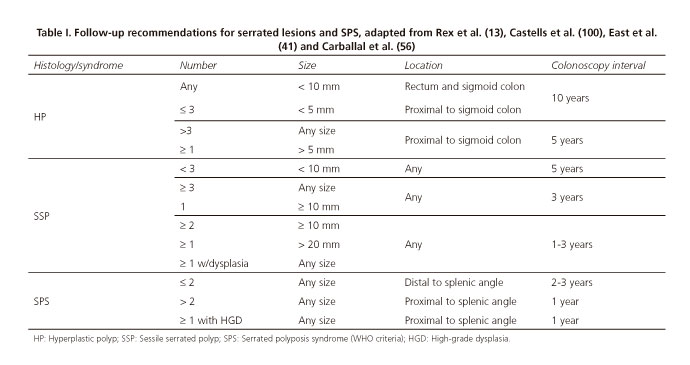
While consensus guidelines have been recently reported, specifically addressing serrated lesion follow-up (13), CRC screening guidelines have usually included them in a similar manner to common adenomatous lesions (99). Overall, expert consensus suggests that CRC risk increases with lesion number and size, SSP versus HP histological subtype, and location in the proximal colon. Although available scientific evidence is scarce regarding serrated lesion progression time and CRC risk, the above-listed variables have been considered when follow-up intervals are suggested by expert groups (13), as summarized in table I.
References
1. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathol 2007;50(1):113-30. DOI: 10.1111/j.1365-2559.2006.02549.x. [ Links ]
2. Li D, Jin C, McCulloch C, Kakar S, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009;104(3):695-702. DOI: 10.1038/ajg.2008.166. [ Links ]
3. Makinen MJ. Colorectal serrated adenocarcinoma. Histopathol 2007;50(1):131-50. DOI: 10.1111/j.1365-2559.2006.02548.x. [ Links ]
4. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterol 2010;138(6):2088-100. DOI: 10.1053/j.gastro.2009.12.066. [ Links ]
5. Huang CS, Farraye FA, Yang S, et al. The clinical significance of serrated polyps. Am J Gastroenterol 2011;106(2):229-40;quiz 41. DOI: 10.1038/ajg.2010.429. [ Links ]
6. Bateman AC, Shepherd NA. UK guidance for the pathological reporting of serrated lesions of the colorectum. J Clin Pathol 2015;68(8):585-91. DOI: 10.1136/jclinpath-2015-203016. [ Links ]
7. Szylberg L, Janiczek M, Popiel A, et al. Serrated polyps and their alternative pathway to the colorectal cancer: a systematic review. Gastroenterol Res Pract 2015;2015:573814. DOI: 10.1155/2015/573814. [ Links ]
8. Boparai KS, Mathus-Vliegen EM, Koornstra JJ, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: A multicentre cohort study. Gut 2010;59(8):1094-100. DOI: 10.1136/gut.2009.185884. [ Links ]
9. O'Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006;30(12):1491-501. DOI: 10.1097/01.pas.0000213313.36306.85. [ Links ]
10. Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterol 2010;139(5):1497-502. DOI: 10.1053/j.gastro.2010.06.074. [ Links ]
11. Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: A prospective study of patients undergoing colonoscopy. Gastroenterol 2006;131(5):1400-7. DOI: 10.1053/j.gastro.2006.08.038. [ Links ]
12. Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150(1):1-8. DOI: 10.7326/0003-4819-150-1-200901060-00306. [ Links ]
13. Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol 2012;107(9):1315-29;quiz 4:30. DOI: 10.1038/ajg.2012.161. [ Links ]
14. Singh H, Turner D, Xue L, et al. Risk of developing colorectal cancer following a negative colonoscopy examination: Evidence for a 10-year interval between colonoscopies. JAMA 2006;295(20):2366-73. DOI: 10.1001/jama.295.20.2366. [ Links ]
15. Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer - A morphological, mucin and lectin histochemical study. Pathol 1992;24(4):233-42. DOI: 10.3109/ 00313029209068874. [ Links ]
16. O'Brien MJ, Zhao Q, Yang S. Colorectal serrated pathway cancers and precursors. Histopathol 2015;66(1):49-65. DOI: 10.1111/his.12564. [ Links ]
17. Snover D AD, Burt RW. Serrated polyps of the colon and rectum and serrated polyposis. En: Bosman FT CF, Hruban RH, Theise ND, eds. World Health Organization classification of tumours pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2010. [ Links ]
18. Hazewinkel Y, De Wijkerslooth TR, Stoop EM, et al. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy 2014;46(3):219-24. [ Links ]
19. Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011;9(1):42-6. DOI: 10.1016/j.cgh.2010.09.013. [ Links ]
20. Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut 1992;33(11):1508-14. DOI: 10.1136/gut.33.11.1508. [ Links ]
21. Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: A necropsy study in Liverpool. Gut 1982;23(10):835-42. DOI: 10.1136/gut.23.10.835. [ Links ]
22. Hasegawa S, Mitsuyama K, Kawano H, et al. Endoscopic discrimination of sessile serrated adenomas from other serrated lesions. Oncol Lett 2011;2(5):785-9. [ Links ]
23. Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol 1990;14(6):524-37. DOI: 10.1097/00000478-199006000-00003. [ Links ]
24. Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterol 1996;110(3):748-55. DOI: 10.1053/gast.1996.v110.pm8608884. [ Links ]
25. Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011;42(1):1-10. DOI: 10.1016/j.humpath.2010.06.002. [ Links ]
26. Rotondano G, Bianco MA, Cipolletta L, et al. Prevalence and characteristics of serrated lesions of the colorectum in Italy: A multicentre prospective cohort study. Dig Liver Dis 2015;47(6):512-7. DOI: 10.1016/j.dld.2015.03.005. [ Links ]
27. Carr NJ, Mahajan H, Tan KL, et al. Serrated and non-serrated polyps of the colorectum: Their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol 2009;62(6):516-8. DOI: 10.1136/jcp.2008.061960. [ Links ]
28. Butterly L, Robinson CM, Anderson JC, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: Results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2014;109(3):417-26. DOI: 10.1038/ajg.2013.442. [ Links ]
29. Bouwens MW, Van Herwaarden YJ, Winkens B, et al. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy 2014;46(3):225-35. DOI: 10.1055/s-0034-1364936. [ Links ]
30. Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010;105(12):2656-64. DOI: 10.1038/ajg.2010.315. [ Links ]
31. Kahi CJ, Li X, Eckert GJ, et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc 2012;75(3):515-20. DOI: 10.1016/j.gie.2011.08.021. [ Links ]
32. Burgess NG, Pellise M, Nanda KS, et al. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut 2015;65:437-46. DOI: 10.1136/gutjnl-2014-308603. [ Links ]
33. Uraoka T, Higashi R, Horii J, et al. Prospective evaluation of endoscopic criteria characteristic of sessile serrated adenomas/polyps. J Gastroenterol 2015;50(5):555-63. DOI: 10.1007/s00535-014-0999-y. [ Links ]
34. Ishigooka S, Nomoto M, Obinata N, et al. Evaluation of magnifying colonoscopy in the diagnosis of serrated polyps. World J Gastroenterol 2012;18(32):4308-16. DOI: 10.3748/wjg.v18.i32.4308. [ Links ]
35. Kimura T, Yamamoto E, Yamano HO, et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol 2012;107(3):460-9. DOI: 10.1038/ajg.2011.457. [ Links ]
36. Burke CA, Snover DC. Sessile serrated adenomas and their pit patterns: We must first see the forest through the trees. Am J Gastroenterol 2012;107(3):470-2. DOI: 10.1038/ajg.2011.468. [ Links ]
37. Torlakovic EE, Gómez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol 2008;32(1):21-9. DOI: 10.1097/PAS.0b013e318157f002. [ Links ]
38. Crockett SD, Snover DC, Ahnen DJ, et al. Sessile serrated adenomas: An evidence-based guide to management. Clin Gastroenterol Hepatol 2015;13(1):11-26e1. DOI: 10.1016/j.cgh.2013.10.035. [ Links ]
39. Bettington M, Walker N, Rosty C, et al. Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol 2014;38(2):158-66. DOI: 10.1097/PAS.0000000000000103. [ Links ]
40. De Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc 2013;77(4):617-23. DOI: 10.1016/j.gie.2012.10.018. [ Links ]
41. East JE, Vieth M, Rex DK. Serrated lesions in colorectal cancer screening: Detection, resection, pathology and surveillance. Gut 2015;64(6):991-1000. DOI: 10.1136/gutjnl-2014-309041. [ Links ]
42. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370(14):1298-306. DOI: 10.1056/NEJMoa1309086. [ Links ]
43. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362(19):1795-803. DOI: 10.1056/NEJMoa0907667. [ Links ]
44. Chetty R, Hafezi-Bakhtiari S, Serra S, et al. Traditional serrated adenomas (TSAs) admixed with other serrated (so-called precursor) polyps and conventional adenomas: A frequent occurrence. J Clin Pathol 2015;68(4):270-3. DOI: 10.1136/jclinpath-2014-202827. [ Links ]
45. Wiland HOt, Shadrach B, Allende D, et al. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol 2014;38(9):1290-7. [ Links ]
46. Sano Y, Saito Y, Fu K-I, et al. Efficacy of magnifying chromoendoscopy for the differential diagnosis of colorectal lesions. Dig Endosc 2005;17(2):105-16. DOI: 10.1111/j.1443-1661.2005.00483.x. [ Links ]
47. Limketkai BN, Lam-Himlin D, Arnold MA, et al. The cutting edge of serrated polyps: A practical guide to approaching and managing serrated colon polyps. Gastrointest Endosc 2013;77(3):360-75. DOI: 10.1016/j.gie.2012.11.013. [ Links ]
48. Kim MJ, Lee EJ, Suh JP, et al. Traditional serrated adenoma of the colorectum: Clinicopathologic implications and endoscopic findings of the precursor lesions. Am J Clin Pathol 2013;140(6):898-911. DOI: 10.1309/AJCPDJC9VC5KTYUS. [ Links ]
49. Kalady MF, Jarrar A, Leach B, et al. Defining phenotypes and cancer risk in hyperplastic polyposis syndrome. Dis Colon Rectum 2011;54(2):164-70. DOI: 10.1007/DCR.0b013e3181fd4c15. [ Links ]
50. Hazewinkel Y, López-Cerón M, East JE, et al. Endoscopic features of sessile serrated adenomas: Validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc 2013;77(6):916-24. DOI: 10.1016/j.gie.2012.12.018. [ Links ]
51. Van Herwaarden YJ, Verstegen MH, Dura P, et al. Low prevalence of serrated polyposis syndrome in screening populations: A systematic review. Endoscopy 2015;47(11):1043-9. DOI: 10.1055/s-0034-1392411. [ Links ]
52. Moreira L, Pellise M, Carballal S, et al. High prevalence of serrated polyposis syndrome in FIT-based colorectal cancer screening programmes. Gut 2013;62(3):476-7. DOI: 10.1136/gutjnl-2012-303496. [ Links ]
53. Biswas S, Ellis AJ, Guy R, et al. High prevalence of hyperplastic polyposis syndrome (serrated polyposis) in the NHS bowel cancer screening programme. Gut 2013;62(3):475. DOI: 10.1136/gutjnl-2012-303233. [ Links ]
54. Bisgaard ML, Fenger K, Bulow S, et al. Familial adenomatous polyposis (FAP): Frequency, penetrance, and mutation rate. Hum Mutat 1994;3(2):121-5. DOI: 10.1002/humu.1380030206. [ Links ]
55. Boparai KS, Reitsma JB, Lemmens V, et al. Increased colorectal cancer risk in first-degree relatives of patients with hyperplastic polyposis syndrome. Gut 2010;59(9):1222-5. DOI: 10.1136/gut.2009.200741. [ Links ]
56. Carballal S, Rodríguez-Alcalde D, Moreira L, et al. Colorectal cancer risk factors in patients with serrated polyposis syndrome: A large multicenter study. Gut 2015. DOI: 10.1016/S0016-5085(15)31872-2. [ Links ]
57. Guarinos C, Sánchez-Fortun C, Rodríguez-Soler M, et al. Clinical subtypes and molecular characteristics of serrated polyposis syndrome. Clin Gastroenterol Hepatol 2013;11(6):705-11;quiz e46. DOI: 10.1016/j.cgh.2012.12.045. [ Links ]
58. Walker RG, Landmann JK, Hewett DG, et al. Hyperplastic polyposis syndrome is associated with cigarette smoking, which may be a modifiable risk factor. Am J Gastroenterol 2010;105(7):1642-7. DOI: 10.1038/ajg.2009.757. [ Links ]
59. Anderson JC, Rangasamy P, Rustagi T, et al. Risk factors for sessile serrated adenomas. J Clin Gastroenterol 2011;45(8):694-9. DOI: 10.1097/MCG.0b013e318207f3cf. [ Links ]
60. Chow E, Lipton L, Lynch E, et al. Hyperplastic polyposis syndrome: Phenotypic presentations and the role of MBD4 and MYH. Gastroenterol 2006;131(1):30-9. DOI: 10.1053/j.gastro.2006.03.046. [ Links ]
61. Win AK, Walters RJ, Buchanan DD, et al. Cancer risks for relatives of patients with serrated polyposis. Am J Gastroenterol 2012;107(5):770-8. DOI: 10.1038/ajg.2012.52. [ Links ]
62. Hazewinkel Y, Koornstra JJ, Boparai KS, et al. Yield of screening colonoscopy in first-degree relatives of patients with serrated polyposis syndrome. J Clin Gastroenterol 2015;49(5):407-12. DOI: 10.1097/MCG.0000000000000103. [ Links ]
63. Balaguer F, Pellise M. Colorectal cancer: Serrated polyposis - Should we screen first-degree relatives? Nat Rev Gastroenterol Hepatol 2014;11(6):333-4. DOI: 10.1038/nrgastro.2014.61. [ Links ]
64. Oquinena S, Guerra A, Pueyo A, et al. Serrated polyposis: Prospective study of first-degree relatives. Eur J Gastroenterol Hepatol 2013;25(1):28-32. DOI: 10.1097/MEG.0b013e3283598506. [ Links ]
65. Edelstein DL, Axilbund JE, Hylind LM, et al. Serrated polyposis: Rapid and relentless development of colorectal neoplasia. Gut 2013;62(3):404-8. DOI: 10.1136/gutjnl-2011-300514. [ Links ]
66. Carvajal-Carmona LG, Howarth KM, Lockett M, et al. Molecular classification and genetic pathways in hyperplastic polyposis syndrome. J Pathol 2007;212(4):378-85. DOI: 10.1002/path.2187. [ Links ]
67. Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterol 2010;138(6):2044-58. DOI: 10.1053/j.gastro.2010.01.054. [ Links ]
68. Lage P, Cravo M, Sousa R, et al. Management of Portuguese patients with hyperplastic polyposis and screening of at-risk first-degree relatives: A contribution for future guidelines based on a clinical study. Am J Gastroenterol 2004;99(9):1779-84. DOI: 10.1111/j.1572-0241.2004.30178.x. [ Links ]
69. Chan TL, Zhao W, Leung SY, et al. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res 2003;63(16):4878-81. [ Links ]
70. Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 2004;53(8):1137-44. DOI: 10.1136/gut.2003.037671. [ Links ]
71. Leggett BA, Devereaux B, Biden K, et al. Hyperplastic polyposis: Association with colorectal cancer. Am J Surg Pathol 2001;25(2):177-84. DOI: 10.1097/00000478-200102000-00005. [ Links ]
72. Abraham J, Núñez-Álvarez Y, Hettmer S, et al. Lineage of origin in rhabdomyosarcoma informs pharmacological response. Genes Dev 2014;28(14):1578-91. DOI: 10.1101/gad.238733.114. [ Links ]
73. Hazewinkel Y, Reitsma JB, Nagengast FM, et al. Extracolonic cancer risk in patients with serrated polyposis syndrome and their first-degree relatives. Fam Cancer 2013;12(4):669-73. DOI: 10.1007/s10689-013-9643-x. [ Links ]
74. Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355(24):2533-41. DOI: 10.1056/NEJMoa055498. [ Links ]
75. Harewood GC, Sharma VK, De Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003;58(1):76-9. DOI: 10.1067/mge.2003.294. [ Links ]
76. Kim JS, Kang SH, Moon HS, et al. Impact of bowel preparation quality on adenoma identification during colonoscopy and optimal timing of surveillance. Dig Dis Sci 2015;60(10):3092-9. DOI: 10.1007/s10620-015-3737-2. [ Links ]
77. Anderson JC, Butterly LF, Robinson CM, et al. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: Data from the New Hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointest Endosc 2014;80(3):463-70. DOI: 10.1016/j.gie.2014.03.021. [ Links ]
78. Oh CH, Lee CK, Kim JW, et al. Suboptimal bowel preparation significantly impairs colonoscopic detection of non-polypoid colorectal neoplasms. Dig Dis Sci 2015;60(8):2294-303. DOI: 10.1007/s10620-015-3628-6. [ Links ]
79. Hurlstone DP, Cross SS, Slater R, et al. Detecting diminutive colorectal lesions at colonoscopy: A randomized controlled trial of pan-colonic versus targeted chromoscopy. Gut 2004;53(3):376-80. DOI: 10.1136/gut.2003.029868. [ Links ]
80. Lapalus MG, Helbert T, Napoleon B, et al. Does chromoendoscopy with structure enhancement improve the colonoscopic adenoma detection rate? Endoscopy 2006;38(5):444-8. DOI: 10.1055/s-2006-925265. [ Links ]
81. Pohl J, Schneider A, Vogell H, et al. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: A randomized two-centre trial. Gut 2010;60(4):485-90. DOI: 10.1136/gut.2010.229534. [ Links ]
82. Kaminski MF, Hassan C, Bisschops R, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2014;46(5):435-49. DOI: 10.1055/s-0034-1365348. [ Links ]
83. Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterol 2012;143(3):599-60 e1. DOI: 10.1053/j.gastro.2012.05.006. [ Links ]
84. Kumar S, Fioritto A, Mitani A, et al. Optical biopsy of sessile serrated adenomas: Do these lesions resemble hyperplastic polyps under narrow-band imaging? Gastrointest Endosc 2013;78(6):902-9. DOI: 10.1016/j.gie.2013.06.004. [ Links ]
85. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterol 2012;143(3):844-57. DOI: 10.1053/j.gastro.2012.06.001. [ Links ]
86. Hewett DG, Huffman ME, Rex DK. Leaving distal colorectal hyperplastic polyps in place can be achieved with high accuracy by using narrow-band imaging: An observational study. Gastrointest Endosc 2012;76(2):374-80. DOI: 10.1016/j.gie.2012.04.446. [ Links ]
87. Hazewinkel Y, Tytgat KM, Van Eeden S, et al. Incidence of colonic neoplasia in patients with serrated polyposis syndrome who undergo annual endoscopic surveillance. Gastroenterol 2014;147(1):88-95. DOI: 10.1053/j.gastro.2014.03.015. [ Links ]
88. Leonard DF, Dozois EJ, Smyrk TC, et al. Endoscopic and surgical management of serrated colonic polyps. Br J Surg 2011;98(12):1685-94. DOI: 10.1002/bjs.7654. [ Links ]
89. Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterol 2013;144(1):74-80e1. DOI: 10.1053/j.gastro.2012.09.043. [ Links ]
90. Morales SJ, Bodian CA, Kornacki S, et al. A simple tissue-handling technique performed in the endoscopy suite improves histologic section quality and diagnostic accuracy for serrated polyps. Endoscopy 2013;45(11):897-905 DOI: 10.1055/s-0033-1344435. [ Links ]
91. Williams JG, Pullan RD, Hill J, et al. Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis 2013;15(Supl 2):1-38. DOI: 10.1111/codi.12262. [ Links ]
92. Kedia P, Waye JD. Colon polypectomy: A review of routine and advanced techniques. J Clin Gastroenterol 2013;47(8):657-65. DOI: 10.1097/MCG.0b013e31829ebda7. [ Links ]
93. Lee CK, Shim JJ, Jang JY. Cold snare polypectomy vs. cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: A prospective randomized study. Am J Gastroenterol 2013;108(10):1593-600. DOI: 10.1038/ajg.2013.302. [ Links ]
94. Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc 2012;76(2):255-63. DOI: 10.1016/j.gie.2012.02.060. [ Links ]
95. Khashab M, Eid E, Rusche M, et al. Incidence and predictors of "late" recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc 2009;70(2):344-9. DOI: 10.1016/j.gie.2008.10.037. [ Links ]
96. Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterol 2011;140(7):1909-18. DOI: 10.1053/j.gastro.2011.02.062. [ Links ]
97. Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: Results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 2015;64(1):57-65. DOI: 10.1136/gutjnl-2013-305516. [ Links ]
98. Repici A, Hassan C, De Paula Pessoa D, et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: A systematic review. Endoscopy 2012;44(2):137-50. DOI: 10.1055/s-0031-1291448. [ Links ]
99. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58(3):130-60. DOI: 10.3322/CA.2007.0018. [ Links ]
![]() Correspondence:
Correspondence:
Alberto Herreros de Tejada Echanojáuregui.
Service of Digestive Diseases. IDIPHIM.
Hospital Universitario Puerta de Hierro Majadahonda.
C/ Manuel de Falla, 1.
28222 Majadahonda.
Madrid, Spain
e-mail: albertoherreros@yahoo.com
Received: 26-10-2015
Accepted: 13-09-2016