Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.9 Madrid sep. 2017
https://dx.doi.org/10.17235/reed.2017.4693/2016
Cost-effectiveness of a hepatitis B virus screening strategy to prevent reactivation in patients with hematologic neoplasms
Coste-efectividad de una estrategia de cribado del virus de la hepatitis B para prevenir la reactivación en pacientes con neoplasia hematológica
Javier Crespo1, Rafael Esteban2, Covadonga Torres3, Itziar Oyagüez3, Miguel Ángel Casado3 and María Buti2
1Hospital Universitario Marqués de Valdecilla. Santander, Spain.
2Hospital Universitario Vall d'Hebrón. Barcelona, Spain.
3Pharmacoeconomics & Outcomes Research Iberia (PORIB). Madrid, Spain
ABSTRACT
Introduction: The effectiveness of a screening strategy for the detection of a hepatitis B virus (HBV) infection followed by prophylaxis in order to prevent HBV reactivation was assessed in patients with hematologic neoplasms.
Material and methods: A decision tree was developed to compare the cost and effectiveness (prevented reactivations) over an 18 month period of a screening strategy prior to chemotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) with a non-screening approach. HBsAg+ (hepatitis B surface antigen) and/or anti-HBc+ (antibodies to hepatitis B core antigen) and HBV-DNA+ patients received oral antiviral prophylaxis with tenofovir disoproxil (245 mg once daily) from chemotherapy baseline until one year after chemotherapy completion. Non-screened patients received tenofovir in case of a reactivation. Model probabilities were obtained from the literature. The total cost (€, 2015) included: antiviral prophylaxis, R-CHOP, screening tests (HBsAg, anti-HBc, HBV-DNA) and liver function tests. Drug therapy costs were estimated using ex-factory prices with mandatory deductions. The incremental cost-effectiveness ratio (ICER) was calculated in order to assess the cost-effectiveness of this intervention in terms of cost per reactivation averted versus no screening.
Results: In a hypothetical cohort of 1,000 patients, screening prevented 7.36 reactivations when compared to the non-screening approach (14.9 versus 22.3). Total cost/patient (including €8,282 for R-CHOP) was €8,584 for the screening strategy and €8,449 for the non-screening approach. The ICER for screening versus non-screening was €18,376/prevented reactivation.
Conclusion: HBV screening followed by oral antiviral prophylaxis yielded more health benefits than non-screening, reducing HBV reactivation in patients with hematologic neoplasms on chemotherapy.
Key words: Screening. Hepatitis B virus. Reactivation. Neoplasm. Cost-effectiveness.
RESUMEN
Introducción: se analizó la efectividad de una estrategia de cribado de la infección por el virus de la hepatitis B (VHB) con consiguiente profilaxis en pacientes con neoplasia hematológica para evitar la reactivación del VHB.
Material y métodos: se utilizó un árbol de decisión para comparar costes y eficacia (reactivaciones evitadas), en 18 meses, de una estrategia con cribado previo a quimioterapia con R-CHOP (rituximab, ciclofosfamida, doxorrubicina, vincristina y prednisona) versus una estrategia sin cribado. Los pacientes HBsAg+ (antígeno de superficie de la hepatitis B) y/o antiHBc+ (anticuerpos del núcleo de la hepatitis B) y ADN-VHB+ recibieron profilaxis antiviral con tenofovir disoproxil desde el inicio de la quimioterapia hasta un año después de su finalización. Los pacientes sin cribado recibieron tenofovir en caso de reactivación. Las probabilidades del modelo se obtuvieron de la literatura. El coste total (€, 2015) incluyó: profilaxis antiviral, R-CHOP, pruebas de cribado (HBsAg, antiHBc y ADN-VHB) y función hepática. El coste farmacológico se calculó con el precio de venta al laboratorio aplicando la deducción obligatoria.
Resultados: en una cohorte hipotética de 1.000 pacientes, el cribado evitó 7,36 reactivaciones frente a la estrategia sin cribado (14,9 versus 22,3). El coste total/paciente (incluyendo 8.282 € de coste de R-CHOP) fue de 8.584 € para la estrategia con cribado y 8.449 € para la estrategia sin cribado. La relación coste-efectividad incremental del cribado versus la estrategia sin cribado fue de 18.376 €/reactivación evitada.
Conclusión: el cribado de la infección por el VHB permite implementar una profilaxis antiviral en pacientes con alto riesgo de reactivación, disminuyendo la frecuencia de dichas reactivaciones en pacientes con neoplasias hematológicas en tratamiento quimioterapia, con un coste incremental aceptable.
Palabras clave: Cribado. Hepatitis B. Reactivación. Neoplasia. Coste-efectividad.
Introduction
Hepatitis B virus (HBV) infection represents a worldwide public health concern; there are approximately 240 million people with a chronic infection (1).
HBV reactivation is defined as any return or increase in HBV-DNA (> 1 log or > 20,000 IU/mL) in a patient with a current or prior HBV infection. HBV reactivation includes a wide range of clinical manifestations, from a mild, transient, silent hepatitis to fulminant and life-threatening forms. The notable heterogeneity of hepatitis B outcome depends on the interaction between the host's immune system and the virus's ability to evade immune mechanisms (2). HBV reactivation was first described over 35 years ago (3), with a higher risk for patients with hematologic malignancies as compared to other neoplasms. However, it has also been reported in patients receiving immunomodulators and/or biologics, immunosuppressants prior to solid organ transplantation and patients with hepatocarcinoma undergoing hepatic chemoembolization. Multiple reports have shown HBV reactivation in various clinical settings. This has become relevant during the last few years as a result of new drugs with a powerful immunosuppressive effect such as rituximab (4,5). In the meta-analysis of 21 studies reported by Kazt et al., 24-100% of patients with HBV reactivation developed acute hepatitis with a highly variable HBV-related mortality of 0-50% (6). As a consequence of HBV reactivation, chemotherapy or immunosuppressive therapy may be delayed or even discontinued and this may have an adverse clinical impact on overall survival (7). In the same meta-analysis, 10-19% of patients had their treatment withdrawn.
In transplanted patients, HBV reactivation increases mortality and graft loss risk. As shown by a meta-analysis of nine studies with 971 patients, the risk is higher when corticosteroids are administered and the risk level is at its highest with regimens combining corticosteroids and rituximab (8). This is particularly true for patients undergoing hematopoietic stem cell transplantation and patients receiving chemotherapy for lymphoma, especially of the non-Hodgkin type (5,7,9-14). In addition, survival in cancer patients recovering from HBV reactivation may be impaired by changes to the scheduled chemotherapy regimen (15).
Three randomized, controlled clinical trials have been performed (13,16,17) with lamivudine as a prophylaxis for Asian HBsAg+ patients scheduled to receive chemotherapy for lymphoma or hepatocarcinoma. The use of prophylaxis lamivudine was associated with a much lower incidence of HBV reactivations when compared to control subjects. In Spain, a multicenter study found that prophylactic tenofovir significantly reduced the incidence and severity of HBV reactivation in association with rituximab chemotherapy (18).
Since chronic infection is usually asymptomatic and commonly associated with normal ALT levels, infected individuals can only be reliably identified by testing for the hepatitis B surface antigen (HBsAg) and antibodies to the hepatitis B core antigen (anti-HBc). Therefore, it is advisable to analyze HBV infection markers before immunosuppressive therapy (1). However, a study performed in the USA found that only 14% of oncologists regularly screened patients (19). Similarly, only 14% of patients in a Toronto hospital were tested for HBsAg before chemotherapy (20).
Furthermore, some recent publications conclude that the evidence is insufficient to establish the benefits or risks of routine screening for a chronic HBV infection in cancer patients scheduled to receive immunosuppressive therapy (21). While preventive antiviral therapy has proven financially acceptable in HBsAg+ patients, information on the cost-effectiveness of HBV screening is scarce (15) and no data are available from Spain. Therefore, the aim of this study was to estimate HBV screening cost-effectiveness in patients with hematologic malignancies before rituximab-based chemotherapy from the perspective of the National Health System.
Methods
An excel-based model was designed that was structured like a decision tree in order to estimate the effectiveness and cost of two potential strategies, HBV screening and prophylaxis with tenofovir disoproxil fumarate (TDF) versus no screening. The study was performed in a hypothetical cohort of 1,000 patients with hematologic cancer before initiating chemotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). The analysis was carried out from a National Health System perspective over an 18 month period (Fig. 1).
Effectiveness was assessed by considering the number of reactivations prevented by the screening versus the non-screening approach. The various probabilities of each strategy (reactivations, hepatitis and death) in each situation reflected the best scientific evidence available following a thorough review of the literature. The estimated prevalence of HBsAg+ was 1.3% and the prevalence of anti-HBc+ in HBsAg- patients was 11.1%. Table 1 details all the probabilities in the model with their respective references.
A numeric parameter known as the incremental cost-effectiveness ratio (ICER) was used to assess cost-effectiveness. Results are expressed as extra cost per incremental benefit unit achieved with the screening strategy versus the non-screening approach. The number of patients who needed to be screened to prevent reactivation was used as an efficacy variable. Therefore, the result should be interpreted as the additional cost that screening would represent in order to prevent one reactivation.
In the screening arm, HBsAg and anti-HBc testing was performed prior to chemotherapy. In patients with positive HBsAg and/or anti-HBc, DNA from the HBV (HBV-DNA) was assessed every other month in the absence of reactivation and every month if reactivation occurred. Patients at a high risk of reactivation (HBsAg+ and/or anti-HBc+ plus HBV-DNA+) received prophylaxis with TDF starting one week before chemotherapy onset until one year after chemotherapy finished.
In the non-screening arm, only delayed antiviral therapy (i.e., therapy at the time of reactivation diagnosis) was considered. From a chronological viewpoint, reactivation may occur after the initial dose of immunosuppressive and/or chemotherapeutic treatment or several months after immunosuppresive therapy was discontinued when the immune system had recovered its ability to detect the increased viral replication that occurred during the immunosuppressed stage. Based on the available evidence, it was assumed that HBV reactivation in patients with hematologic malignancy would peak at four months after R-CHOP onset (20,22,23).
Total cost estimates for each strategy included chemotherapy and antiviral drug costs, screening costs, liver function monitoring costs and costs related to clinical events (hepatitis and death). Drug costs were estimated according to ex-factory prices established in the "Catálogo de Medicamentos del Consejo General de Colegios Oficiales de Farmacéuticos" (24) and then applying the mandatory deduction as established by the Spanish Royal Decree 8/2010 (25). Antiviral therapy included the oral administration of TDF of one daily 245-mg tablet (26). R-CHOP chemotherapy cost was estimated for six 21-day cycles at a standard dose: rituximab, 375 mg/m2 on day 1 (27); cyclophosphamide, 750 mg/m2 on day 1 of every cycle (28); hydroxidaunorubicin: 50 mg/m2 on day 1 of every cycle (29); Oncovin®, 1.4 mg/m2 (max. 2 mg) on day 1 of every cycle (30); and prednisone, 100 mg on days 1 and 5 of every cycle (31). The body surface area considered was 1.7 m2 (32,33).
Liver function tests, including transaminase and bilirubin levels, were performed every two months. In the case of HBV reactivation, monthly monitoring was performed. Unitary costs for screening measurements and liver function monitoring were obtained from a national cost database (34). With regard to hepatitis management costs, the estimated costs of hepatitis B management in a study performed in various European countries including Spain were used. In addition, the average estimates from 2014 by the Spanish Ministry of Health, Social Services and Equality were used that corresponded to the minimum basic data set (MBDS) discharge registry from 2012 (35) for the following ICD-9 codes: 070 (viral hepatitis), 070.30 (viral hepatitis B without hepatic coma, acute or unspecified without hepatitis delta), 070.32 (chronic viral hepatitis B without hepatic coma without hepatitis delta), 573.3 (hepatitis, unspecified), 571.40 (chronic hepatitis, unspecified), and 571.49 (other chronic hepatitis) (36).
The cost of hepatic mortality was obtained from the literature (37) and updated according to the CPI inter-annual variation rate reported by the Statistics National Institute (38).
In order to assess the uncertainty of the results, several one-way sensitivity analyses (SA) were performed. New scenarios were set up and the following parameters were modified. The time to reactivation since R-CHOP chemotherapy onset was modified from four to five months (SA1).
The number of R-CHOP cycles considered was modified from six to eight cycles (a regimen also used in standard clinical practice) (SA2). With regard to the percentage of HBsAg- subjects, the prevalence was changed from 1.3% to 0.7% (prevalence in the general population) (SA3). The percentage of anti-HBc+ subjects was reduced by 25%, i.e., changing the prevalence from 11.10% to 8.32% (SA4). The probability of non-hepatic death in HBsAg+ patients with no reactivation in the screening arm was changed rendering it identical to the non-screening arm, i.e., changing the value from 0.0% to 7.8% (SA5).
All costs were expressed in euros (€) using values from 2015 (Table 2).
Results
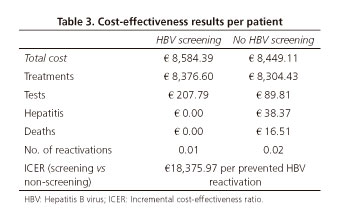
In a hypothetical cohort of 1,000 patients with a hematological neoplasm on R-CHOP therapy, a HBV screening strategy prior to chemotherapy onset would prevent a total of 7.36 reactivations as compared to the non-screening approach during an 18 month period (Table 3).
The total cost per patient was €8,584 in the screening strategy, with R-CHOP chemotherapy accounting for 97.6% of the cost. This total cost per patient was €8,449 for the non-screening approach, with drug costs accounting for 98.3% of the total (Table 3). In other words, the screening strategy represents an incremental cost of €135 per patient, i.e., an additional 1.6% versus non-screening. Furthermore, the contribution of clinical events (hepatitis, death) to the total cost of the screening strategy was 0.0%, whereas this contribution amounted to 0.6% in the non-screening arm.
The resulting ICER was €18,376 per prevented HBV reactivation in the screening versus the non-screening approach over a period of 18 months (Table 3).
One-way SA yielded the following results. With regard to the number of prevented reactivations, no changes occurred in the value obtained for the base case except for the analyses of modified percentages of HBsAg- subjects (SA3) and anti-HBc+ subjects (SA4). The 7.36 prevented reactivations in the base case were reduced to 4.28 and 7.19 for SA3 and SA4, respectively. With regards to the ICER (€18,376 per prevented reactivation with the screening versus non-screening approach in the base case), the values ranged from €16,078.07 for SA4 (modified percentage of anti-HBc+ subjects) to €27,155.06 for SA3 (modified percentage of HBsAg- subjects).
Discussion
The PRESCRIB study assessed the cost-effectiveness of HBV screening in patients with hematologic malignancies prior to chemotherapy onset. This is one of the few studies that explored effectiveness of R-CHOP chemotherapy and the only study to assess cost-effectiveness in Spain. Furthermore, this is the first study to assess effectiveness as a function of prevented reactivation, a clinical parameter usually considered in routine clinical practice.
According to the results obtained, the cost of screening and the associated monitoring of liver function represents an additional cost of €135 per patient over a period of 18 months, with a prevented reactivation rate of 7.36 per 1,000 patients.
Therefore, we can conclude that antiviral prophylaxis after HBV screening reduces reactivation and mortality rates in patients with hematologic neoplasms on chemotherapy. Screening slightly increases overall cost as compared to the non-screening approach. However, screening reduces reactivations, which in turn may result in clinical events such as hepatitis, hepatocellular carcinoma, liver transplantation and hepatic death (39). There is also a factor that is difficult to quantify that has a negative impact on patients: the inability or delay in administering chemotherapy in patients with reactivation.
A recent study in Spain (18) compared the efficacy of TDF prophylaxis versus observation in anti-HBc+ and HBsAg- patients on rituximab therapy for hematologic malignancies. The results were in line with those obtained in the present study and showed that TDF administration prevented HBV reactivation.
Another study (15) assessed the cost-effectiveness of three screening strategies (all patients, only high-risk patients and no screening) in patients with lymphoma over a 12 month period. The study suggested that screening all patients was cost-effective as compared with the other strategies assessed, although the differences were small. However, this study only separated patient clinical profiles according to baseline HBsAg status.
The study by Day et al. (40) also involved a cost-effectiveness analysis in Australia that compared a screening approach to not screening in patients with solid tumors. They concluded that screening was cost-effective for selected patient subpopulations and/or by simplifying screening strategies.
It is interesting to note that the two previously reported cost-effectiveness studies used the same methodology and analyzed data using a decision tree-based model.
The limitations associated with the present study include the short time period of the analysis and the fact that the selection was conditioned by the available evidence of reactivation in this patient profile. A broader time period would allow the identification of significant differences in costs and results between the assessed alternative options, thus rendering the results more valid. In this respect, the natural history of the disease and how it may be modified by the assessed therapies are relevant factors that should be taken into account. Another aspect worth mentioning is that the model assumes that screening does not delay chemotherapy. However, it should be ensured that this is actually the case in routine clinical practice. Furthermore, the prophylactic strategy used (tenofovir) might not be appropriate for all patients and lamivudine could be used as an alternative. Finally, the difficulty in the interpretation of the above data is associated with a lack of a specific threshold of the cost per effectiveness unit achieved, in this case per reactivation prevented.
The public health care system has to cope with a shortage of resources and increasing demands for services. Thus, the healthcare and social benefits of implementing programs and strategies (such as new markers for reactivation [41]) with a prophylactic rather than therapeutic effect should be debated with regard to an adequate efficiency and additional advantages. However, these results show that HBV screening in a population with hematologic malignancies scheduled to receive rituximab-based chemotherapy will identify patients who will benefit from antiviral prophylaxis. This represents an added cost of €135 per patient over an 18 month period versus non-screening, and prevents 7.36 reactivations per 1,000 patients.
Acknowledgements
The authors thank Cándido Hernández from Gilead Sciences for his cooperation in the literature search for the identification of the most relevant papers.
The authors are also grateful to the reviewers of the Revista Española de Enfermedades Digestivas for their comments and proposals during the review process, which allowed an improvement of the quality of the manuscript.
References
1. World Health Organization. Media Centre: Hepatitis B. July 2015. Disponible en: http://www.who.int/mediacentre/factsheets/fs204/en/. [ Links ]
2. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: What we knew in 1981 and what we know in 2005. Hepatol 2006;43(2 Supl 1):S173-81. DOI: 10.1002/hep.20956. [ Links ]
3. Wands JR, Chura CM, Roll FJ, et al. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterol 1975;68:105-12. [ Links ]
4. Hui CK, Cheung WW, Zhang HY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterol 2006;131:59-68. DOI: 10.1053/j.gastro.2006.04.015. [ Links ]
5. Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009;27:605-11. DOI: 10.1200/JCO.2008.18.0182. [ Links ]
6. Katz LH, Fraser A, Gafter-Gvili A, et al. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: Systematic review and meta-analysis. J Viral Hepat 2008; 15:89-102. [ Links ]
7. Francisci D, Falcinelli F, Schiaroli E, et al. Management of hepatitis B virus reactivation in patients with hematological malignancies treated with chemotherapy. Infection 2010;38:58-61. DOI: 10.1007/s15010-009-9019-1. [ Links ]
8. Dong HJ, Ni LN, Sheng GF, et al. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: A meta-analysis. J Clin Virol 2013;57:209-14. DOI: 10.1016/j.jcv.2013.03.010. [ Links ]
9. Marzano A, Angelucci E, Andreone P, et al. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig Liver Dis 2007;39(5):397-408. DOI: 10.1016/j.dld.2006.12.017. [ Links ]
10. Raimondo G, Navarra G, Mondello S, et al. Occult hepatitis B virus in liver tissue of individuals without hepatic disease. J Hepatol 2008;48(5):743-6. DOI: 10.1016/j.jhep.2008.01.023. [ Links ]
11. Kusumoto S, Tanaka Y, Ueda R, et al. Reactivation of hepatitis B virusfollowing rituximab-plus-steroid combination chemotherapy. J Gastroenterol 2011;46(1):9-16. DOI: 10.1007/s00535-010-0331-4. [ Links ]
12. Matsue K, Kimura S, Takanashi Y, et al. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer 2010;116(20):4769-76. DOI: 10.1002/cncr.25253. [ Links ]
13. Lau GK, Yiu HH, Fong DY, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing hemotherapy. Gastroenterol 2003;125(6):1742-9. DOI: 10.1053/j.gastro.2003.09.026. [ Links ]
14. Evens AM, Jovanovic BD, Su YC, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: Meta-analysis and examination of FDA safety reports. Ann Oncol 2011;22(5):1170-80. DOI: 10.1093/annonc/mdq583. [ Links ]
15. Zurawska U, Hicks LK, Woo G, et al. Hepatitis B virus screening before chemotherapy for lymphoma: A cost-effectiveness analysis. J Clin Oncol 2012;30(26):3167-73. DOI: 10.1200/JCO.2011.40.7510. [ Links ]
16. Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatol 2006;43(2):233-40. DOI: 10.1002/hep.21024. [ Links ]
17. Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: A randomized trial. Hepatol 2008;47(3):844-53. DOI: 10.1002/hep.22106. [ Links ]
18. Buti M, Morillas R, Manzano ML, et al. Tenofovir for the prophylaxis of HBV reactivation in anti-HBc-positive patients with hematologic malignancies treated with rituximab - Preliminary results of a randomized study (PREBLIN study). J Hepatol 2014;60(1):S421(P1040). DOI: 10.1016/S0168-8278(14)61200-9. [ Links ]
19. Viganò M, Serra G, Casella G, et al. Reactivation of hepatitis B virus during targeted therapies for cancer and immune-mediated disorders. Expert Opin Biol Ther 2016;16(7):917-26. DOI: 10.1080/14712598.2016.1177017. [ Links ]
20. Pei SN, Chen CH, Lee CM, et al. Reactivation of hepatitis B virus following rituximab-based regimens: A serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol 2010;89(3):255-62. DOI: 10.1007/s00277-009-0806-7. [ Links ]
21. Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: Chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol 2010;28(19):3199-202. DOI: 10.1200/JCO.2010.30.0673. [ Links ]
22. Kim SJ, Hsu C, Song YQ, et al. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: Analysis from the Asia Lymphoma Study Group. Eur J Cancer 2013;49(16):3486-96. DOI: 10.1016/j.ejca.2013.07.006. [ Links ]
23. Li HR, Huang JJ, Guo HQ, et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat 2011;18(12):877-83. DOI: 10.1111/j.1365-2893.2010.01386.x. [ Links ]
24. Base de Datos de Medicamentos del Consejo General de Colegios Farmacéuticos. BotPlus. Disponible en: https://botplusweb.portalfarma.com/. [ Links ]
25. Real Decreto Ley 8/2010 - BOE. Disponible en: www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf DOI: 10.1016/j.janxdis.2010.02.003. [ Links ]
26. Ficha técnica de tenofovir. Disponible en: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/000419/WC500051737.pdf. [ Links ]
27. Ficha técnica de rituximab. Disponible en: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdf. [ Links ]
28. Ficha técnica de ciclofosfamida. Disponible en: https://www.aemps.gob.es/cima/pdfs/es/ft/79065/FichaTecnica_79065.html.pdf. [ Links ]
29. Ficha técnica de hidroxidaunorubicina. Disponible en URL: https://www.aemps.gob.es/cima/pdfs/es/ft/62177/FT_62177.pdf. [ Links ]
30. Ficha técnica de Oncovin. Disponible en: https://www.aemps.gob.es/cima/pdfs/es/ft/71117/FichaTecnica_71117.html.pdf. [ Links ]
31. Ficha técnica de prednisona. Disponible en: https://www.aemps.gob.es/cima/pdfs/es/ft/29724/29724_ft.pdf. [ Links ]
32. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098. PMID 3657876. [ Links ]
33. Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: A height-weight formula validated in infants, children and adults. J Pediatr 1978;93:62-6. [ Links ]
34. Oblikue consulting. eSalud. Disponible en: http://oblikue.com. [ Links ]
35. Ministerio de Sanidad, Servicios Sociales e Igualdad. Instituto de Información Sanitaria. (2014). Registro de altas. CIE9 MC-CMBD 2012. Disponible en: http://pestadistico.inteligenciadegestion.msssi.es/. [ Links ]
36. Brown RE, De Cock E, Colin X, et al. Hepatitis B management costs in France, Italy, Spain, and the United Kingdom. J Clin Gastroenterol 2004;38(10 Supl 3):S169-74. DOI: 10.1097/00004836-200411003-00009. [ Links ]
37. Buti M, Brosa M, Casado MA, et al. Modeling the cost-effectiveness of different oral antiviral therapies in patients with chronic hepatitis B. J Hepatol 2009;51(4):640-6. DOI: 10.1016/j.jhep.2009.04.013. [ Links ]
38. Instituto Nacional de Estadística. Cálculo de variaciones del Índice de Precios de Consumo. Disponible en: http://www.ine.es. [ Links ]
39. Hay AE, Meyer RM. Hepatitis B, rituximab, screening, and prophylaxis: Effectiveness and cost effectiveness. J Clin Oncol 2012;30(26):3155-7. DOI: 10.1200/JCO.2012.43.7509. [ Links ]
40. Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol 2011;29(24):3270-7. DOI: 10.1200/JCO.2011.35.1635. [ Links ]
41. Seto WK, Wong DH, Chan TY, et al. Association of hepatitis B core-related antigen with hepatitis B virus reactivation in occult viral carriers undergoing high-risk immunosuppressive therapy. Am J Gastroenterol 2016;111(12):1788-95. DOI: 10.1038/ajg.2016.436. [ Links ]
42. Álvarez Suárez B, De la Revilla Negro J, Ruiz-Antoran B, et al. Reactivación de la hepatitis B y su impacto clínico actual. Rev Esp Enferm Dig 2010;102(9):542-52. [ Links ]
43. Gutiérrez García ML, Alonso López S, Martín Ríos MD, et al. Hepatitis B virus reactivation in rituximab-treated patients: Incidence and risk factors. Gastroenterol Hepatol 2015;38(1):1-6. DOI: 10.1016/j.gastrohep.2014.05.008. [ Links ]
44. Yeguas A, Turcu V, Núñez J, et al. Profilaxis y reactivación de la hepatitis en la era del rituximab. Experiencia clínica en el Hospital Universitario de Getafe. LIV Reunión de la Sociedad Española de Hematología y Hemoterapia. Salamanca, 18-20 oct 2012. Póster P-008. Disponible en: http://www.sehh.es/images/stories/recursos/2013/comunicaciones_cientificas/2012/LIV_Congreso_SEHH_2012.pdf. [ Links ]
45. Sampedro B, Hernández-López C, Ferrándiz JR, et al. Computerized physician order entry-based system to prevent HBV reactivation in patients treated with biologic agents: The PRESCRIB project. Hepatol 2014;60(1):106-13. DOI: 10.1002/hep.27103. [ Links ]
46. Hsu C, Tsou HH, Lin SJ, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: A prospective study. Hepatol 2014;59(6):2092-100. DOI: 10.1002/hep.26718. [ Links ]
47. Saab S, Dong MH, Joseph TA, et al. Hepatitis B prophylaxis in patients undergoing chemotherapy for lymphoma: A decision analysis model. Hepatol 2007;46(4):1049-56. DOI: 10.1002/hep.21783. [ Links ]
48. Papadopoulos N, Deutsch M, Manolakopoulos S, et al. Is antiviral prophylactic treatment necessaray in HBsAg(-), antiHBc(+) and antiHBs(+) onco-hematologic patients with high or moderate risk for HBV reactivation. European Association for the Study of the Liver. EASL Special conference. Optimal Management of Hepatitis B Virus Infection. Atenas, 25-27 sept 2014. [ Links ]
49. Papadopoulos N, Deutsch M, Manolakopoulos S, et al. Is antiviral prophylactic treatment necessaray in HBsAg(-), antiHBc(+) and antiHBs(+) onco-hematologic patients with high or moderate risk for HBV reactivation. European Association for the Study of the Liver. EASL Special conference. Optimal Management of Hepatitis B Virus Infection. Atenas, 25-27 sept 2014. [ Links ]
![]() Correspondence:
Correspondence:
Itziar Oyagüez.
Pharmacoeoconomics & Outcomes Research Iberia (PORIB).
Paseo Joaquín Rodrigo 4o I.
28224 Pozuelo de Alarcón, Madrid. Spain
e-mail: ioyaguez@porib.com
Received: 29-10-2016
Accepted: 11-04-2017











 texto en
texto en