My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 n.11 Madrid Nov. 2017
https://dx.doi.org/10.17235/reed.2017.4898/2017
Insulin resistance and the metabolic syndrome are related to the severity of steatosis in the pediatric population with obesity
Esther Ubiña-Aznar1, Leopoldo Tapia-Ceballos2, José Miguel Rosales-Zabal3, Rocío Porcel-Chacón2, Francisco Poveda-Gómez4, Carmen Lozano-Calero5, Carmen Ortiz-Cuevas6, Francisco Rivas-Ruiz7, Andrés Sánchez-Cantos3 and Jose María Navarro-Jarabo3
Units of 1Gastroenterology and 6Radiology. Hospital de Alta Resolución de Guadix. Guadix, Granada. Spain.
Departments of 2Pediatrics, 3Gastroenterology, 4Internal Medicine, and 5Radiology. Hospital Costa del Sol. Marbella, Málaga. Spain.
7Research Units. Hospital Costa del Sol and Research Network of Health Services in Chronic Diseases (REDISSEC). Marbella, Málaga. Spain
Author's contributions: Guarantor of the article: JMNJ. Study design: JMNJ, EUA. Manuscript drafting: JMNJ. Data acquisition: EUA, LEP, JMRZ, RPC, FPG, CLC, COC, ASC. Data analysis: JMNJ, EUA, JMRZ. Statistical analyses and interpretation: FRR, JMNJ. Manuscript correction: JMRZ. All authors approval of the submitted and published versions.
ABSTRACT
Background: To determine the factors associated with an increased risk for severe steatosis (SS) and establish the Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) as a screening tool.
Methods: A cross-sectional study was performed in obese children to assess the relationship between the metabolic syndrome (MetS) and glucose metabolism alterations (GMA) and the risk for severe steatosis.
Results: A total of 94 children (51 males) aged from six to 14 years were included. Thirteen children (14.8%) had severe steatosis (SS). The anthropometric variables associated with SS included body mass index (BMI) (SS 34.1 vs non-SS 29.7, p = 0.005), waist circumference (cm) (100 vs 92.5, p = 0.015) and hip circumference (cm) (108 vs 100, p = 0.018). The blood parameters included alanine aminotransferase (ALT) (UI/dl) (27 vs 21, p = 0.002), gamma-glutamil transpeptidase (GGT) (UI/dl) (16 vs 15, p = 0.017), fasting glycemia (mg/dl) (96 vs 88, p = 0.006), fasting insulin (UI/dl) (25 vs 15.3, p < 0.001) and HOMA-IR score (7.1 vs 3.7, p < 0.001). Eighteen children with MetS were found to be at an increased risk for severe steatosis (odds ratio [OR] 11.36, p < 0.001). After receiver operating characteristic (ROC) curve analysis, the best area under the curve (AUC) was obtained for HOMA-R of 0.862. The HOMA-R 4.9 cut-off value had a 100% sensitivity (CI 95%: 96.2-100) and 67.9% specificity (CI 95%: 57.1-78.7) for severe steatosis.
Conclusions: The presence of MetS and glucose metabolism alterations are risk factors for severe steatosis. The 4.9 cut-off value for HOMA-IR may be a risk factor for severe steatosis in obese children.
Key words: Non-alcoholic fatty liver disease. Non-alcoholic steatohepatitis. Liver steatosis. Pediatric obesity. Metabolic syndrome. Insulin resistance.
Introduction
Obesity is a major health problem in the pediatric population and is closely related to the metabolic syndrome (MetS). MetS is defined as the association of several risk factors that are precursors of atherosclerotic cardiovascular disease such as diabetes type 2 or any glucose metabolism alteration, atherogenic dyslipidemia and hypertension (HTA). Insulin resistance plays a crucial role in the etiology of these diseases (1,2). Non-alcoholic fatty liver disease (NAFLD) is considered as the hepatic manifestation of MetS (3-5). As in adults, NAFLD manifests in children as a broad spectrum of lesions ranging from mild steatosis to severe decompensated cirrhosis (6). In recent years, NAFLD has become the most common liver disease in the pediatric population (7,8). Early diagnosis is essential as non-alcoholic steatohepatitis (NASH) is associated with a poor prognosis (9). Screening for NAFLD in children is challenging since the serological parameter generally used, elevated serum transaminase levels, is not cost-effective. Transaminases are usually within normal levels in patients with NAFLD and are only elevated in patients with the most severe forms of NASH (10,11). Radiological imaging is useful for the diagnosis of NAFLD. Steatosis is detectable by ultrasonography present in more than 30% of hepatocytes (12). Other imaging tests such as computed tomography (CT) and especially magnetic resonance imaging (MRI) have a higher sensitivity for NAFLD detection than ultrasonography, as they detect milder fatty accumulations (13). However, access to these imaging tests is limited and entails higher costs.
Children with obesity are a known risk groups for NAFLD. Therefore, the risk factors associated with steatosis in obese children should be identified, especially for the most severe forms of steatosis. Our research group has recently shown that steatosis can be detected by ultrasonography in 34% of children who are overweight or obese (14). The study also showed that the group of patients without steatosis and the group with mild steatosis had similar characteristics. In addition, these groups differed from the group of patients with severe steatosis. This finding suggests that there are two clearly defined profiles of obese children with regard to NAFLD. Although transaminases were within normal levels in most patients, an ALT cut-off value for discarding severe steatosis was identified. This cut-off value could be useful to determine the cost-effectiveness of further examinations for severe steatosis in obese children.
In the same study, the prevalence of MetS and the glucose metabolism profile was examined in a subgroup of patients. The purpose of the current study was to determine the risk factors associated with severe steatosis.
Methods
The study was performed in a subgroup of obese children from a cohort of a previous study that were referred to a childhood obesity unit (14). Glucose homeostasis and MetS characteristics were analyzed to determine the factors associated with severe steatosis confirmed by ultrasonography. Study variables included glucose metabolism alterations (GMA) (such as impaired fasting glucose [IFG] or oral glucose overload [OGO]) and peripheral insulin resistance alterations (IR: fasting insulinemia, blood glucose/insulin index, HOMA-R index or quantitative insulin sensitivity check index [QUICKI]). MetS was defined as the combination of at least three of the following alterations: central obesity (waist circumference > 90th percentile [P90]), (15) any GMA (GMA, OGO, diabetes mellitus [DM]), hypertriglyceridemia > 110 mg/dl, HDL-cholesterol < 40 mg/dl and arterial hypertension (AHT: diastolic or systolic blood pressure > P90) (16).
Obesity was determined using a standard stadiometer (SECA 713, SECA Hamburg, Germany). Body mass index (BMI) was calculated as weight/height (kg/m2). Results were expressed as absolute values after adjustment to the 99th percentile of age and gender (17).
The study was approved by the Ethics Committee of the Hospital Costa del Sol. Informed consent was obtained from all parents and legal guardians.
The primary endpoint was the presence of severe steatosis defined as grade 2-3 steatosis using sonographic criteria (18). Ultrasonography was performed using the General Electric Logiq 400 sonographer (General Electric, Milwaukee, WI, USA). Ultrasound images were reviewed by two independent observers. A diagnosis of steatosis was confirmed only when there were no discrepancies. A comparative analysis was performed of patients without steatosis (grade 0-1) and those with severe steatosis (grade 2-3).
The following independent variables were examined with regard to their possible association with the presence of steatosis: age, sex, anthropometric measures (weight in kg, height in cm, BMI [weight/height in m2] in percentiles according to age and sex defined for the intervals ≥ P95-P98 and ≥ P99), waist circumference in cm and percentiles of age and sex for the intervals < P90 and ≥ P90, hip circumference in cm and percentiles of age and sex for the intervals < P90 and ≥ P90, blood parameters (triglycerides, total cholesterol, high density lipoprotein [HDLc] and low density lipoprotein [LDL] in mg/dl), aminotransferases (alanine aminotransferase [ALT], normal range < 40 Ul/l, and aspartate transaminase [AST], normal range < 40 UI/l) and gamma-glutamil transpeptidase (G-GT) (normal range < 50 U/l). Insulin-resistance status was determined by the insulin resistance score (HOMA-IR) using the following formula: glycemia (mmol/l) x fasting insulin (μUI/ml) / 22.5. The QUICKI was calculated as follows: 1 / (log fasting insulin [μUI/ml] + log fasting glucose [mg/dl]).
Glycemic homeostasis was detected by an oral glucose overload defined as 1.75 gr of glucose/kg (maximum 75 grs); glycemia was measured before and 120 minutes after glucose overload.
Statistical analysis
A descriptive analysis of measures of central tendency and spread (median and interquartile range [IQR]) for quantitative variables and frequency distribution for qualitative variables was performed. Subgroup comparison for the outcome variable (absence vs presence of severe steatosis) was conducted using the Mann-Whitney U test for differences in quantitative variables and the Chi-squared test for qualitative variables. An OR was calculated for both tests for the presence of severe steatosis with 95% confidence levels (CI 95%). The AUC with 95% CI was computed for misbalanced quantitative variables. The diagnostic performance (sensitivity, specificity and positive and negative predictive values) of the variables with the best AUC according to the best cut-off value selected was also assessed. Finally, inter-observer agreement with regard to the diagnosis of steatosis and grade of severity was assessed using the Kappa statistic (k). A p < 0.05 was considered as statistically significant.
Results
In total, 94 children (51 males and 43 females) were included in the study. All subjects had a BMI > P99, a median age of eleven years and an IQR of 2.5. A total of 35 (37.2%) had some grade of steatosis, which was severe in 13 cases (grade 2-3). The results of the group comparison (severe steatosis [SE] vs no steatosis [NE]) are summarized in (table 1. Statistically significant differences were observed in BMI (0.005), waist circumference (0.015) and hip circumference (0.018), ALT (0.002), GGT (0.017), fasting glycemia (0,006), insulinemia (p < 0.001), glucose/insulin ratio (< 0.001), HOMA-IR score (p < 0.001), QUICKI index (p < 0.001) and the presence of MetS (< 0.001). ALT or AST levels were high in five patients, all with severe steatosis (p < 0.001).
Considering that insulin resistance (IR) is defined as a HOMA-IR score ≥ 3.8, IR was observed in 44 children (46.8%). IR was significantly more frequent in the group with severe steatosis (OR 18.37, p = 0.006).
A subgroup of 18 patients (19.1%) met the diagnostic criteria for MetS. Gender-based differences were not observed (23.5% males vs 15.4% females, p = 0.24). A GMA was detected in 11.7% of cases (fasting glycemia: 8.5%; OGO: 5.3%; DM: 1.1%). It is noteworthy that GMAs were more frequent in the group without steatosis (20% vs 6.8%, OR 3.43; CI 95: 0.927-12.740, p = 0.054). Eight of 13 patients with severe steatosis had MetS, whereas only ten of 81 patients without steatosis had MetS (p < 0.001).
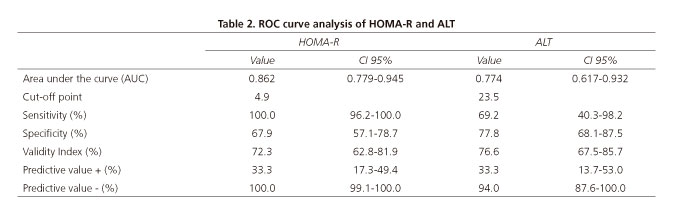
AUC was estimated for these variables in order to determine cut-off values for the prediction of severe steatosis. An ALT cut-off value of 23.5 had a better sensitivity and specificity, with a negative predictive value of 94% (CI 95%: 87.6-100). The HOMA-IR cut-off value of 4.9 yielded the best AUC (0.862), with 100% sensitivity (CI 95%: 96.2-100) and 67.9% specificity (CI 95%: 57.1-78.7), and a negative predictive value of 100% (Table 2).
Discussion
In this study, 13.8% of patients within the cohort of 94 obese children referred to the childhood obesity unit had severe steatosis confirmed by ultrasonography (grade 2-3). Severe steatosis was associated with alterations in anthropometric parameters, the presence of glucose metabolism alterations (GMA: fasting hyperglycemia, abnormal OGO curve or diabetes) with an OR 4.70 (CI 95 1.15-19.24), and IR parameter alterations (fasting insulin: OR 1.07, glucose/insulin index: OR 0.53, HOMA-IR: OR 1.31, and QUICKI index: OR 0.52).
The results obtained suggest that severe steatosis is closely related to glucose homeostasis alterations, which is consistent with the observations from previous studies in adults and children. The study by Santoro et al. (20), including a multiethnic population of 229 adolescent with a mean age of 12.8 years, also found that IR was associated with the grade of steatosis as determined by MRI. In addition, steatosis was also related to elevated plasma citokeratin-18 levels, which is a surrogate marker of inflammatory activity (NASH). However, this relationship was only found in the subgroup of Hispanic and Caucasian children and not in African children, where liver disease mechanisms seem to be mediated by genetic factors related to lipid metabolism (21).
The influence of IR in the development of NASH has also been confirmed in patients with a biopsy-based diagnosis. Patton et al. (22) analyzed a cohort of 254 children who underwent a biopsy for NAFLD, of whom 25.6% had MetS. IR severity was significantly related to a confirmed diagnosis of NASH. The ORs for IR markers (fasting insulinemia: 1.052, p = 0.01; HOMA-IR: 1.283, p = 0.007; and QUICKI: 0.786, p < 0.001) were similar to those obtained in this study.
As previously mentioned, NAFLD is the most common liver alteration in the pediatric population (8,23). Screening for NAFLD has been recently recommended for obese children with a BMI > P95 and overweight children (BMI ≥ P85 and < P94) with risk factors. ALT is the best screening tool for NAFLD in children (8) since ALT is associated with a higher risk for NASH (24,25). In contrast to other studies, plasma ALT levels were not a sensitive parameter for NAFLD in children. The findings in this study are consistent with those of previous studies. Therefore, we can conclude that any of the surrogate IR markers are a cost-effective and easily applicable clinical tool to identify obese children at an increased risk of severe steatosis.
This study confirms that NAFLD is the hepatic manifestation of MetS as the risk for severe steatosis increased significantly in obese children with MetS, with an OR of 11.36 (CI 95% 3.1-41.6). A strong correlation between NASH severity and MetS was demonstrated by Patton et al. This study showed that the risk for MetS is higher in children with severe steatosis (OR 2.58, p = 0.001) and in children with a confirmed diagnosis of NASH (OR 4.07, p = 0.002) (22). Similarly, the case-control study of 150 obese children by Schwimmer et al. (3) showed that the presence of MetS increased the risk for NAFLD, with an OR of 5 (CI 2.6-9.7). Furthermore, Love-Osborne K et al. (27) found that the occurrence of MetS was associated with a higher risk for AMG, elevated ALT levels and greater steatosis severity confirmed by ultrasonography. These results are consistent with those obtained in this study. However, the inclusion criteria of the former study was a fasting insulin level > 25 UI/ml and the population studied was older (15.8 years) than the population of this study (eleven years).
Other studies have revealed a relationship between MetS and an increased risk for fibrosis. Biopsies performed in 120 children in the study by Manco et al. (28) showed that grade of fibrosis was the only histological feature of NASH that was significantly related to MetS. However, this study was conducted in a selected population with persistently elevated or fluctuating transaminase levels, which contrasts with the inclusion criteria in this study, where patients were selected according to the presence of obesity and normal transaminase levels.
There is no robust evidence with regard to the prognosis of NAFLD during childhood. The association between NAFLD and an increased cardiovascular risk is alarming given the prevalence of NAFLD in children and adolescents. The population can develop arteriosclerosis during adolescence. Indeed, autopsy-based studies have revealed early signs of arteriosclerosis during childhood and adolescence, especially when associated risk factors are present (29,30). There is evidence of decreased survival in adolescents and children with NAFLD, with a 13.8 fold lower survival with respect to controls without NAFLD (9). Some studies have observed a relationship between NAFLD severity confirmed by ultrasonography and carotid atherosclerosis in obese children; this increased risk is independent of any anthropometric and metabolic characteristics (5). In the same way, an increased thickness of the carotid was found to be significantly related to IR and NAFLD (31). NAFLD may serve as an independent marker of cardiovascular risk in the pediatric population (3).
In this study, HOMA-IR scores and ALT levels were found to be the most effective predictors of severe steatosis. An ALT value of 23.5 was a very effective negative predictor factor of severe steatosis. The data obtained are consistent with those recently reported by Rehm et al. (32) in a study in 82 adolescent girls. A similar ALT cut-off value (24 UI/dl) was found to have a negative predictive value of 93% and a positive predictive value of 34%.
Nevertheless, the variable with the highest predictive value for severe steatosis was the HOMA-IR score. A cut-off value of 4.9 yielded an AUC of 0.86, with a 100% negative predictive value and a 33% positive predictive value. In agreement with our results, Rehm also found that a HOMA-IR cut-off value of 6.7 yielded an excellent AUC of 0.87 and a negative predictive value of 95%, with a positive predictive value of 53%, a specificity of 87% and a sensitivity of 77%. It is noteworthy that the performance of HOMA-IR reported in the Rehm study was very similar to that found in this study. Differences in the cut-off value for HOMA-IR may be explained by the fact that only female patients with a broad age range (11-22) were included in the study.
A limitation of this study is that the diagnosis of steatosis was based on ultrasound tests. The gold standard for the diagnosis and prognosis of NFALD is liver biopsy. However, the performance of liver biopsies is limited due to the cost and associated risk, and they are only performed in selected patients with persistently elevated transaminases. To overcome this limitation, ultrasound images were reviewed by two independent observers. Inter-observer agreement (19) with regard to the presence of steatosis was confirmed by a k value of 0.82 (very good agreement) for the diagnosis of steatosis and 0.73 (good agreement) for the degree of steatosis (p = 0.0001).
With regard to the study population, another limitation is that the results obtained can only be applied to obese children with normal transaminase levels. Therefore, the results obtained should be validated for non-obese children with a broader age range that also includes adolescents. Similarly, a prospective long-term follow-up study should be conducted in a cohort of obese children at risk of severe steatosis in order to assess the impact of severe steatosis in the early development of cardiovascular lesions (for instance, lesions in the carotid intima).
In conclusion, ALT and HOMA-IR are effective markers to screen for severe liver steatosis in obese children. The identification of obese children at an increased risk of severe steatosis would lead to a further examination and a closer follow-up.
Declarations
All the authors certify that they have no affiliations or involvement with any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers' bureaus, membership, employment, consultancies, stock ownership, or other equity interest and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
No financial support was received for this study.
All patients provided written informed consent and all data (including clinical, demographic and test results) were coded and deposited in the original clinical database. The Ethics Committee of the Hospital Costa del Sol approved the protocol. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice.
References
1. Cook S, Auinger P, Li C, et al. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999-2002. J Pediatr 2008;152(2):165-70. DOI: 10.1016/j.jpeds.2007.06.004. [ Links ]
2. Lee S, Bacha F, Gungor N, et al. Comparison of different definitions of pediatric metabolic syndrome: Relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr 2008;152(2):177-84. DOI: 10.1016/j.jpeds.2007.07.053. [ Links ]
3. Schwimmer JB, Pardee PE, Lavine JE, et al. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008;118(3):277-83. DOI: 10.1161/CIRCULATIONAHA.107.739920. [ Links ]
4. Demircioglu F, Kocyigit A, Arslan N, et al. Intima-media thickness of carotid artery and susceptibility to atherosclerosis in obese children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 2008;47(1):68-75. DOI: 10.1097/MPG.0b013e31816232c9. [ Links ]
5. Pacifico L, Cantisani V, Ricci P, et al. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res 2008;63(4):423-27. DOI: 10.1203/PDR.0b013e318165b8e7. [ Links ]
6. Loomba R, Sirling CB, Schwimmer JB, et al. Advances in pediatric nonalcoholic fatty liver disease. Hepatol 2009;50(4):1282-93. DOI: 10.1002/hep.23119. [ Links ]
7. Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118(4):1388-93. DOI: 10.1542/peds.2006-1212. [ Links ]
8. Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). JPGN 2017;64(2):319-34. DOI: 10.1097/MPG.0000000000001482. [ Links ]
9. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut 2009;58(11):1538-44. DOI: 10.1136/gut.2008.171280. [ Links ]
10. Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis 2004;8(3):549-58. DOI: 10.1016/j.cld.2004.04.010. [ Links ]
11. Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr 2000;30(1):48-53. DOI: 10.1097/00005176-200001000-00017. [ Links ]
12. Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123(3):745-50. DOI: 10.1053/gast.2002.35354. [ Links ]
13. Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: A noninvasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatr Radiol 2001;31(11):806-9. DOI: 10.1007/s002470100547. [ Links ]
14. Navarro-Jarabo JM, Ubiña-Aznar E, Tapia-Ceballos L, et al. Hepatic steatosis and severity-related factors in obese children. J Gastroenterol Hepatol 2013;28(9):1532-8. DOI: 10.1111/jgh.12276. [ Links ]
15. SEEDO'2000 consensus for the evaluation of overweight and obesity and the establishment of criteria for therapeutic intervention. Sociedad Española para el Estudio de la Obesidad. Med Clin (Barc) 2000;115(15):587-97. [ Links ]
16. Grupo Cooperativo Español para el estudio de los factores de riesgo cardiovascular en la infancia y adolescencia en España. Estudio RICARDIN II: valores de referencia. An Pediatr (Barc) 1995;43:11-7. [ Links ]
17. Serra Majem L, Ribas Barba L, Aranceta Bartrina J, et al. Childhood and adolescent obesity in Spain. Results of the enKid study (1998-2000). Med Clin (Barc) 2003;121(19):725-32. DOI: 10.1016/S0025-7753(03)74077-9. [ Links ]
18. Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292(6512):13-5. DOI: 10.1136/bmj.292.6512.13. [ Links ]
19. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159-74. DOI: 10.2307/2529310. [ Links ]
20. Santoro N, Feldstein AE, Enoksson E, et al. The association between hepatic fat content and liver injury in obese children and adolescents: Effects of ethnicity, insulin resistance, and common gene variants. Diabetes Care 2013;36(5):1353-60. DOI: 10.2337/dc12-1791. [ Links ]
21. Guerrero R, Veha GL, Grundy SM, et al. Ethnic differences in hepatic steatosis: An insulin resistance paradox? Hepatol 2009;49(3):791-801. DOI: 10.1002/hep.22726. [ Links ]
22. Patton HM, Yates K, Unalp-Arida A, et al. Association between metabolic syndrome and liver histology among children with nonalcoholic fatty liver disease. Am J Gastroenterol 2010;105(9):2093-102. DOI: 10.1038/ajg.2010.152. [ Links ]
23. Chalasani N, Younossi Z, Lavine J, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatol 2012;55(6):2005-23. DOI: 10.1002/hep.25762. [ Links ]
24. Barlow SE, Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007;120(S4):S164-92. DOI: 10.1542/peds.2007-2329C. [ Links ]
25. August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: An endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93(12):4576-99. DOI: 10.1210/jc.2007-2458. [ Links ]
26. Targher G, Bertolini L, Padovani R, et al. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30(5):1212-8. DOI: 10.2337/dc06-2247. [ Links ]
27. Love-Osborne KA, Nadeau KJ, Sheeder J, et al. Presence of the metabolic syndrome in obese adolescents predicts impaired glucose tolerance and non-alcoholic fatty liver disease. J Adolesc Health 2008;42(6):543-8. DOI: 10.1016/j.jadohealth.2007.11.136. [ Links ]
28. Manco M, Marcellini M, Devito R, et al. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008;32(2):381-7. DOI: 10.1038/sj.ijo.0803711. [ Links ]
29. Stary HC. Lipid and macrophage accumulations in arteries of children and the development of atherosclerosis. Am J Clin Nutr 2000;72(5S):1297S-306S. [ Links ]
30. Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338(23):1650-6. DOI: 10.1056/NEJM199806043382302. [ Links ]
31. Kelishadi R, Cook SR, Amra B, et al. Factors associated with insulin resistance and non-alcoholic fatty liver disease among youths. Atherosclerosis 2009;204(2):538-43. DOI: 10.1016/j.atherosclerosis.2008.09.034. [ Links ]
32. Rehm JL, Connor EL, Wolfgram PM, et al. Predicting hepatic steatosis in a racially and ethnically diverse cohort of adolescent girls. J Pediatr 2014;165(2):319-25. DOI: 10.1016/j.jpeds.2014.04.019. [ Links ]
![]() Correspondence:
Correspondence:
José Miguel Rosales-Zabal.
Gastroenterology Unit.
Hospital Costa del Sol.
A-7, km 187.
29603 Marbella, Málaga. Spain
e-mail: jmiguelrz@hotmail.com
Received: 13-02-2017
Accepted: 14-07-2017