Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.109 no.12 Madrid dic. 2017
https://dx.doi.org/10.17235/reed.2017.5063/2017
Value and innovation of direct-acting antivirals: long-term health outcomes of the strategic plan for the management of hepatitis C in Spain
Juan Turnes1, Raquel Domínguez-Hernández2 and Miguel Ángel Casado2
1Department of Gastroenterology and Hepatology. Complejo Hospitalario Universitario de Pontevedra. Instituto de Investigación Sanitaria Galicia Sur. Pontevedra, Spain.
2Pharmacoeconomics & Outcomes Research Iberia (PORIB). Madrid, Spain
Authors' conflict of interest: Juan Turnes received unconditional funding from Gilead Sciences for the development of the analysis. Raquel Domínguez-Hernández and Miguel Ángel Casado are employees of Pharmacoeconomics & Outcomes Research Iberia, a consultancy firm specializing in the economic evaluation of healthcare interventions, which has received unconditional funding from Gilead Sciences for the development of the analysis.
ABSTRACT
Objective: To assess the long-term healthcare costs and health outcomes in association with the access to new direct-acting antivirals (DAAs), during the first year of the National Strategic Plan for Chronic Hepatitis C (SPCHC) in patients with chronic hepatitis C (CHC) in Spain.
Methods: A decision tree and a lifetime Markov model were developed to simulate the natural history, morbidity, and mortality of a cohort of 51,900 patients with CHC before (pre-DAA strategy) and after (post-DAA strategy) access to DAAs, following SPCHC approval. The percentage of patients treated, transition probabilities, disease management costs, health state utility values, sustained virologic response rates and treatment costs were obtained from the literature and published data from Spain. The results were expressed in terms of costs (€, 2016), quality-adjusted life years (QALYs) and prevention of clinical events, with an annual discount rate of 3%.
Results: The post-DAA strategy would prevent 8,667 cases of decompensated cirrhosis, 5,471 cases of hepatocellular carcinoma, 1,137 liver transplants and 9,608 liver-related deaths. The cohort of 51,900 patients would require investments of 1,606 and 1,230 million euros with the post-DAA and pre-DAA strategies, respectively. This would produce 819,674 and 665,703 QALYs.
Conclusions: The use of new DAA-based treatments in CHC patients during the first year after the implementation of the SPCHC significantly reduced long-term morbidity and mortality and increased quality of life; demonstrating that this plan is an efficient use of public health resources.
Key words: Direct action antiviral agents. Chronic hepatitis C. Strategic plan for hepatitis C management. Social value. Innovation. Efficacy.
Introduction
Studies performed before the implementation of the National Strategic Plan for Chronic Hepatitis C (SPCHC) estimated that the prevalence of hepatitis C virus (HCV) infection in Spain was 1.6%. This corresponds to approximately 475,000 people, of whom only 40% were diagnosed (1). Chronic hepatitis C (CHC) is characterized by the development of liver fibrosis, which leads to liver cirrhosis in up to 25% of patients (2). In most cases, this progression is slow and completely asymptomatic. However, once liver cirrhosis develops, the annual probability of developing clinically decompensated cirrhosis (DC) is 4% and the probability of developing hepatocellular carcinoma (HCC) is 1.5% (3). These conditions could eventually require liver transplantation (LT) or result in death. Additionally, the delayed diagnosis and treatment of CHC facilitates viral transmission among the population.
From 1990 to 2013, the worldwide mortality rates related to cirrhosis and HCC caused by hepatitis C increased by 67% and 291%, respectively (4). Therefore, HCC resulting from CHC represents a major health burden (5) and is now among one of the eight pathologies that cause over 100,000 annual deaths worldwide. In Spain, the estimated number of total deaths attributable to HCV infection was 4,342 in 2000 from a total of 360,391 deaths (6), accounting for 1.5% of the deaths in that year. A premature death represents a quantifiable loss of life years. In 2006, the burden of CHC in Spain in terms of years of life lost ranged from 63,753 to 73,790 years (7). Therefore, CHC was the seventh leading cause of years of life lost. Moreover, when life years with disability are added to years of life lost, the resulting disability-adjusted life years (DALYs) loss ranges from 71,122 to 81,336 (7).
The efficacy of oral antiviral therapies based on pegylated IFNα (peg-IFN) that have been developed during the last decade has had a relatively low impact on the burden of HCV infection (8). Modelling studies conducted using data from a Spanish population evaluated outcomes following the use of peg-IFN and ribavirin (peg-IFN/RBV) or triple therapy with an HCV protease inhibitor (boceprevir or telaprevir) and peg-IFN/RBV. A study showed that there were reductions in mortality (ranging from 8.1% to 10.3%) and cirrhosis incidence (ranging from 13.8% to 18.4%) only in patients infected with genotype 1 HCV (9).
The burden of CHC in Spain requires the use of innovative health interventions with improved efficacy and safety, such as new direct-acting antiviral agents (DAAs) in peg-IFN free regimens. These therapeutics have achieved > 95% infection cure rates (10-12) and have the potential to rapidly reduce the burden of the disease.
In Spain, the institutional response to the introduction of new DAAs regimens aimed to ensure access to treatment in a way that remains sustainable for the health system. These goals were pursued via the development and rapid implementation of the SPCHC in 2015 (13). The SPCHC states that all patients are eligible for high-effective DAA therapy. However, due to the extent of the disease in Spain, it was decided that patients with advanced liver fibrosis (including stages F2-F4), patients on transplant waiting lists and those with recurrent HCV infection should be prioritized for these treatments. Other European countries have implemented similar measures to strategically prioritize patients for DAAs treatment by categorizing them into subgroups (14).
Given that economic resources are limited and that the sustainability of public health systems must be ensured in decision-making, it is necessary to evaluate the clinical evidence and cost-effectiveness of innovative therapeutics. When assessing treatment priorities, the principal question that must be addressed in an economic evaluation is whether the overall costs of an intervention are sufficiently counterbalanced by the health outcomes achieved. These factors should always be compared with the standard alternatives that are available at the time of decision-making. In addition, patient clinical characteristics (15,16) must be considered as well as the economic and National Healthcare System of each country (17). To address these issues, we first assessed the extent to which new DAAs prevent the hepatic complications of disease progression (e.g., DC, HCC, LT, and liver disease-related mortality). It is also important to evaluate the corresponding direct and indirect costs and the healthcare and non-healthcare resources used by patients with and without a sustained virologic response (SVR) to HCV therapy; such as patients without access to treatment or for whom treatment was not effective (18).
The aim of this study was to evaluate long-term health costs and outcomes during the first year (2015) after application of the SPCHC using new DAAs (post-DAA) in patients infected with CHC (≥ F2) from the perspective of the National Health System in Spain. These data were compared to those obtained when using previously available regimens (pre-DAA).
Methods
We compared incremental long-term health outcomes and costs generated throughout the patient's life on the use of new DAAs during the first year (2015) after implementation of SPCHC (post-DAA strategy) versus those produced using previously available regimens based on peg-interferon plus ribavirin and telaprevir or boceprevir prior to the SPCHC (pre-DAA strategy), in CHC patients.
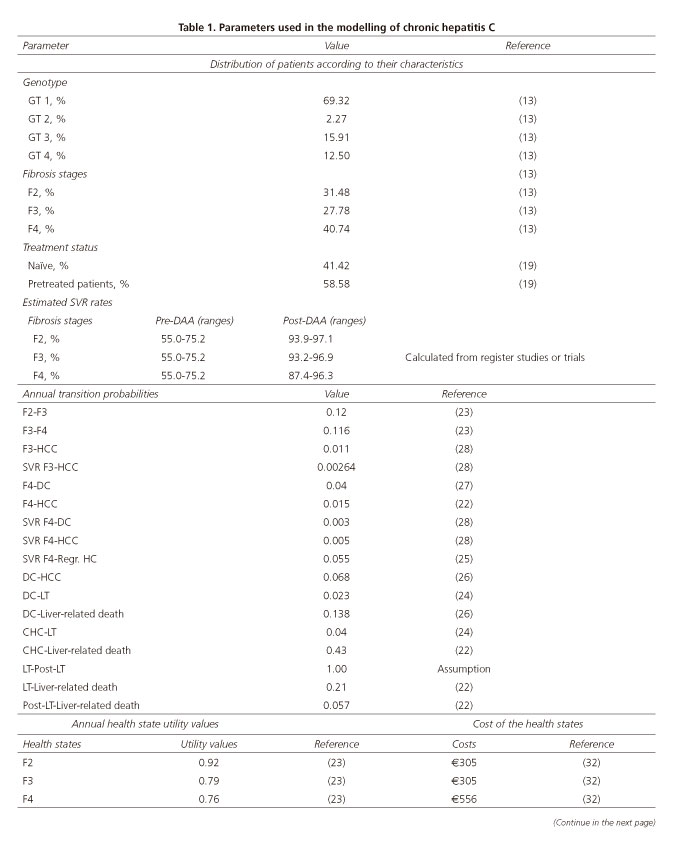
The analysis included 51,900 CHC patients with significant or advanced fibrosis (≥ stage F2) (13) with an average age of 52 (19). All patients were treated according to the European Medicines Agency (EMA) approved combinations of DAAs IFN free regimens (13). A decision tree was designed and patients were separated based on whether they were treated or untreated and were proportionally distributed according to their clinical characteristics (genotype, degree of fibrosis, treatment-naïve or previously treated status) (Table 1). The tree was used to calculate the global SVR rates for each fibrosis stage, regardless of genotype. In total, 73% of the patients were treated using the post-DAA strategy and 19% were treated with the pre-DAA strategy (20). SVR rates according to clinical characteristics were obtained from clinical trials or the most relevant studies of each treatment included in the analysis (21). The remaining patient clinical characteristics included in the decision tree were obtained from the literature and published Spanish data and are shown in table 1.
A lifetime Markov model was developed based on previously validated models (19,21) in order to project and analyze disease progression; during which patients could have transitioned annually from one stage to another depending on the probabilities of developing DC or HCC, undergoing LT or moving to post-LT status. Disease progression was monitored until death. Annual probabilities were obtained from the literature (22,24,29) (Table 1). SVR patients with stage F2 fibrosis were considered as no longer at risk of disease progression, whereas those with stage F3 or F4 fibrosis remained at risk of developing DC or HCC. In addition, according to the published data, the achievement of an SVR following treatment in some cirrhotic patients could lead to the fibrosis regression (23). Therefore, this transition was considered in the simulation of disease for patients in the SVR F4 state. The patient quality of life for each health state is represented by utility measures obtained from the literature (24). Disutility was also considered during treatment administration (30) (Table 1).
With regard to the pharmacological cost of the pre-DAA strategy, an average cost of €15,003 per patient was calculated (31) within the Spanish Health System, including the application of corresponding deductions. For the post-DAA strategy, the overall pharmacological cost of treating all patients with CHC in 2015 with the new DAA strategies was €1,094 million (32). For both strategies, the average cost of treatment monitoring was €1,257 for the post-DAA strategy and €2,371 for the pre-DAA strategy. These costs included different treatment durations (8, 12, 24, or 48 weeks) and were calculated from published data (33). The annual health costs associated with each health state were obtained from published data (30,33). All costs are expressed in Euros and were updated in 2016.
Analysis of health costs and outcomes
Health outcomes were measured in terms of quality-adjusted life years (QALYs), which were calculated by multiplying the years of life gained with each strategy by the utility value. This measure reflects a patients' preference for each health state (34). The incremental cost was calculated as the difference in total cost (drug, monitoring, and management of complications) between one strategy and another.
In order to calculate the present value of future costs and health outcomes and compare them in terms of a net present value, a discount rate of 3% was applied (35). However, because there is debate regarding whether health outcomes and cost should be discounted, and if this discount is applied at what rate and point in time, data on costs and health outcomes without the discount rate (0%) applied have also been included in some cases (36).
In order to provide a monetary estimate of the societal value of quality of life adjusted survival gain, the number of incremental QALYs obtained with the post-DAA strategy versus the pre-DAA strategy was multiplied by different values of cost per QALY or willingness to pay (37), such as 20,000, 25,000 and 30,000 euros per QALY (37,38).
Sensitivity analysis
Different one-way sensitivity analyses (OWSA) were performed for each minimum and maximum value for the parameters that generated a greater uncertainty of the analyzed results. These parameters included the SVR rates for each fibrosis stage considering the ranges of values obtained in the decision tree (87.4-93.2% for post-DAA and 55.0-75.2% for pre-DAA) and assuming the ranges of values of the percentage of patients treated (66-100% for post-DAA and 17-21% for pre-DAA), drug costs (± 20%) and LT costs (± 20%).
Results
Figure 1 shows the overall cost and health outcome expressed as QALYs for the 51,900 patients evaluated. The incremental cost of the post-DAA strategy compared with the pre-DAA strategy is €375 million. For the period in which access to new DAAs was considered, that is, in the first year of SPCHC implementation, 153,970 additional QALYs were obtained with the post-DAA strategy compared to the pre-DAA strategy.
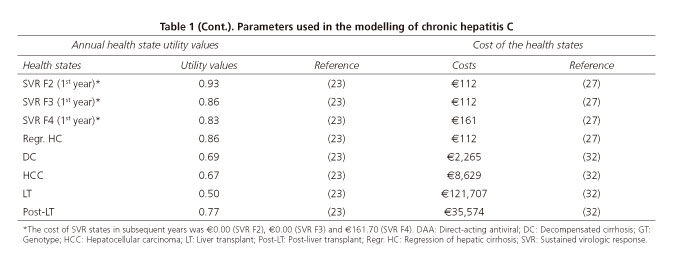
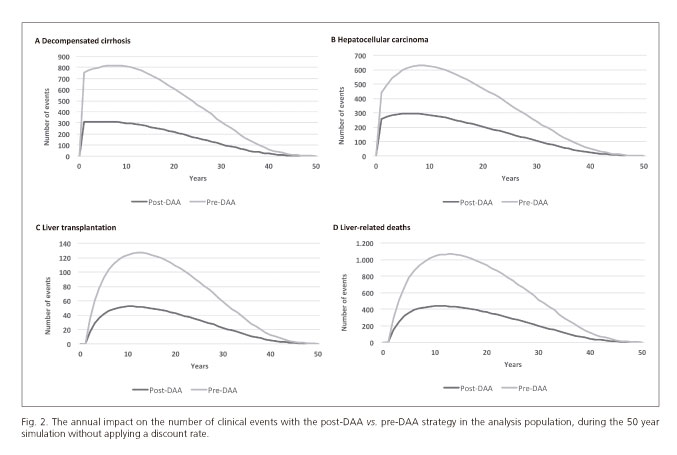
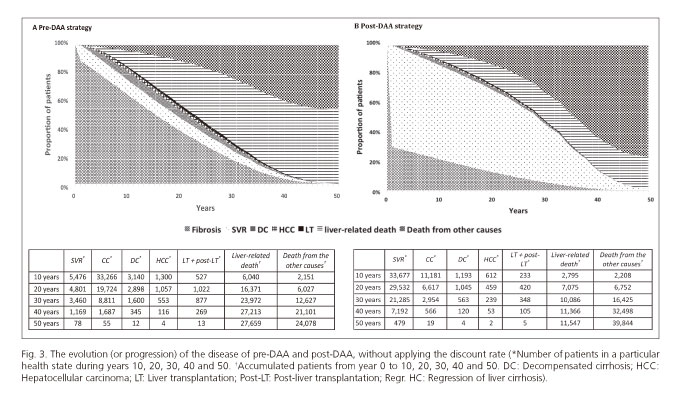
After disease modelling of the cohort, the post-DAA strategy would avoid 8,667 (63%) cases of DC, 5,471 (53%) cases of HCC, 1,137 (59%) LTs and 9,607 (58%) liver disease-related deaths compared to the pre-DAA strategy. This would save approximately €468 million, 84% of these savings would result from the reduced number of LTs and cases of HCC following the post-DAA strategy (Table 2). The number of avoided events and their associated costs without a discount rate applied are shown in table 2 and figures 2 A-D.
After running the model for 10 years without applying a discount rate and using the post-DAA strategy, 64% of the total cohort (51,900) had achieved a SVR, 2.2% had DC, 1.1% had HCC and 5.3% had died from liver-related causes. Significantly fewer patients from the total cohort achieved a SVR at 10 years (10.5%) with the pre-DAA strategy. The proportion of patients with DC or HCC increased to 6.0% and 2.5%, respectively and the mortality rate associated with liver disease was 11.6% (Fig. 3 A and B).
Estimation of the monetary value of a QALY (cost per QALY: 20,000, 25,000 and 30,000 euros)
The monetary value of the incremental QALYs achieved with the post-DAA strategy versus the pre-DAA strategy within the cohort using the number of incremental QALYs of the baseline case (Fig. 1) ranged from 3,079 to 4,619 million euros with a value between 20,000 and 30,000 euros per QALY (Fig. 4).
Sensitivity analysis results
The QALY values ranged between 135,607 and 220,701 additional QALYs when the OWSA was used to compare the post-DAA and pre-DAA strategies. The incremental cost ranged between 156 and 594 million Euros. Drug costs had the greatest influence on the results in the post-DAA strategy, which amounted to €218 million. In contrast, drug cost had a lower impact in the pre-DAA strategy (€29 million). As all patients in the post-DAA strategy group were treated, the increment QALY gain was 220,701 which was associated with an incremental cost of €531 million. Modification of the percentage of patients treated in the pre-DAA strategy did not significantly alter the results of the analysis. When minimum values of SVR rates were considered, 141,381 and 160,285 QALYs were obtained for the post-DAA and pre-DAA strategies, respectively. Variations in the cost of transplantation were not associated with significant variations in incremental cost (€27 million).
Discussion
The introduction of oral DAA regimens to treat CHC has changed the management of this disease (40). The high efficacy and safety of DAAs allow any HCV-infected patient to be treated and eventually cured (40). The present analysis evaluated long-term health outcomes and costs following the implementation of the SPCHC in 2015. The results demonstrate that the prevention of clinical events related to liver disease and the costs associated with their management and the increased quality-adjusted life years compensate for the financial investment in drug costs.
Health resources are limited and must be managed in a manner consistent with the needs of a population to ensure the sustainability of a health system. One way to manage these resources is using value-based health systems, which have a fundamental objective of achieving the best health outcome for patients at an acceptable and sustainable cost. For this to occur, measures must be adopted based on value and this value is defined from the result obtained per monetary unit invested. These measures include estimating disease cost, improving and innovating health technologies, ensuring patient access to innovative treatments with disruptive changes over health outcomes and improving the general health of the population.
It should be noted that the costs of CHC treatment only corresponded to the first year following the implementation of the SPCHC, as the objective of this analysis was to evaluate health outcomes in 2015. The analysis only took into account the investment already made and not the cost of continuing the SPCHC. As more patients are treated, this cost will decrease for various reasons, including reductions in the number of patients eligible for treatment due to the decreased disease prevalence that results from the high treatment efficacy of new DAAs, as well as reductions in treatment costs. This phenomenon has already been reflected in the CHC drug expenditure corresponding to the second half of 2016, which was 63% lower than that in 2015 (41). To measure the impact of drug cost change on analysis results, an OWSA was performed in which drug costs were varied for each treatment strategy (pre-DAA and post-DAA). The results of the analysis showed an incremental cost of €218 million. Therefore, the predicted lower drug cost in the future would reduce the incremental cost and increase the efficiency of the continuation and/or extension of the treatment strategies presented in the SPCHC.
The effectiveness of new DAAs in treating CHC is evident and there is a strong level of evidence supporting their use in clinical practice. However, whether the budgetary impact derived from the use of DAAs translates into a worthwhile investment for health systems needs to be assessed. It is important to consider that patients with CHC who were treated with new DAAs achieved SVR rates of > 95% (10-12,18), indicating that most were cured and that investing in drugs to treat CHC might be worthwhile.
Another important factor associated with value systems is innovation. In this regard, the measurement and comparison of health outcomes with those obtained from previous strategies is essential (42,43). The new DAAs have contributed to an increase in treatment efficacy and effectiveness, a greater tolerability and adherence by patients, a shortening of treatment duration and a simpler dosage regimen, which is particularly innovative. However, evaluating the efficiency of innovation requires the estimation and association of the cost of innovation, which is the relationship between the increased SVR rates produced by the new DAAs and their cost in this case (44). This relationship must be established and compared to drugs previously used as standard therapies. In our analysis, the post-DAA strategy implemented in the SPCHC was more cost-effective than the pre-DAA strategy from a clinical standpoint.
The reduced number of LTs for the patients who were cured in 2015 represents a savings of €509 million, which corresponds to an overall saving of 65% of CHC follow-up and complication treatment costs. However, these results may underestimate the actual effect, as new evidence suggests that eliminating HCV in patients already on LT waiting lists might lead to significant improvements in liver function, ultimately resulting in patients being withdrawn from the waiting list (45). A collateral benefit of the reduction in LTs for patients with CHC is the increase in availability of livers for organ donation for other indications (46). In Spain, 36.4% of all LTs in 2015 were performed in cirrhosis patients due to HCV and HCC (47). As the new DAAs prevent CHC from progressing to more advanced stages, according to our analysis, the number of transplants would be expected to be reduced by 46.2% over the next 10 years.
Our analysis also focused on the monetary value of the post-DAA strategy by extrapolating the QALYs achieved by the post-DAA strategy compared to the pre-DAA strategy (48). This approach has been used in other health evaluations such as the study by Van Nuys et al., which evaluated the social benefit in monetary terms from the QALYs obtained with new DAAs and from different values assigned to the efficiency threshold in the treatment of HCV-infected patients in the United States (37). In the present study, the monetary value associated with the increase in QALYs achieved with the post-DAA versus pre-DAA strategy (153,981 QALYs) across the patient cohort ranged from 3,069 to 4,604 million Euros. This far exceeds the cost increase of €375 million.
In the SPCHC, patients who should have preferential access to the new DAAs are defined as those with significant fibrosis (≥ F2) and those whose treatment cannot be deferred. The total number of patients estimated to receive treatment was 51,900, and approximately 73% of these patients were treated in 2015 and the remainder was treated at the beginning of 2016. A more accurate assessment would require real observed data of SVR rates obtained from the SPCHC and the evaluation of access for all infected patients, including those in the initial stages of fibrosis. This study did not include patients in stage F0 or F1 as this was not the objective of the study. Future pharmacoeconomic studies must include all infected patients in Spain in order to evaluate the overall clinical and economic impact of the new policies. Furthermore, as our analysis focused on the current population of patients who had already received treatment, the development and implementation of screening programs for the detection of HCV in high-risk groups in order to enable their access to curative treatment should be examined in future revisions of the SPCHC. On the other hand, the modelling here focused only on the first year following the implementation of the SPCHC, and the extra-hepatic manifestations associated with CHC, which occur irrespective of the stage of liver disease (49) and could increase the total disease burden, have not been taken into account (50). Had these situations been considered, the difference in efficacy and safety between previous and new antivirals would probably have shown a greater clinical benefit by preventing a greater number of liver complications and also extra-hepatic complications within the CHC population.
Another factor that should be considered is the fact that this analysis was conducted from the perspective of the National Health System and not from a societal perspective. Thus, the productivity loss of CHC patients was not considered. This is relevant as the CHC population is mostly a working age population (50). Prior studies on work productivity associated with HCV infection have confirmed that CHC patients show high rates of absenteeism and that their work productivity significantly deteriorates (50). This deterioration does not affect treated and untreated patients equally as different treatments impact to a greater or lesser extent on productivity due to the variability in adverse events. In economic terms, this translates into increased indirect costs resulting from lost working hours as well as increased direct costs due to factors such as increased consumption of health resources (50). These costs may be added to the cost of premature deaths due to CHC, which has been estimated at €1,054 million over a period of three years (51). Only direct costs were incorporated in our analysis and any costs associated with the loss of productivity were excluded. Had these latter costs been included, it would have significantly increased the total cost of the pre-DAA strategy due to the low percentage of patients treated and their poorer health status compared with those treated with the post-DAA strategy.
Conclusion
To ensure the sustainability of the healthcare system, prioritizing care for patients in more advanced stages of disease is crucial and the subsequent establishment of a treatment strategy for all patients, regardless of their fibrosis stage. The present economic evaluation demonstrates that the use of DAA-based treatments for CHC patients in Spain would significantly reduce the morbidity and mortality associated with the disease and thus represents an efficient use of public resources. Despite the initial disbursement allocated to the acquisition of new treatments, the current treatment strategy for the population classified as a priority in the SPCHC (post-DAA) increases long-term societal value and guarantees sustainability of the National Health System.
References
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis virus (HCV) infection with today's treatment paradigm. J Viral Hepat 2014;21(Suppl 1):34-59. DOI: 10.1111/jvh.12248. [ Links ]
2. Lavanchy D. The global burden of hepatitis C. Liver Int 2009;29(Suppl 1):74-81. DOI: 10.1111/j.1478-3231.2008.01934.x. [ Links ]
3. Younossi ZM, Singer ME, Mir HM, et al. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1patients. J Hepatol 2014;60:530-7. DOI: 10.1016/j.jhep.2013.11.009. [ Links ]
4. GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1775-812. [ Links ]
5. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081-8. DOI: 10.1016/S0140-6736(16)30579-7. [ Links ]
6. García-Fulgueiras A, García-Pina R, Morant C, et al. Hepatitis C and hepatitis B-related mortality in Spain. Eur J Gastroenterol 2009;21:895-901. DOI: 10.1097/MEG.0b013e328313139d. [ Links ]
7. García-Fulgueiras A, García-Pina R, Morant C, et al. Burden of disease related to hepatitis C and hepatitis B in Spain: a methodological challenge of an unfolding health problema. J Viral Hepat 2011;18:e453-60. DOI: 10.1111/j.1365-2893.2011.01467.x. [ Links ]
8. Crespo J, Cabezas J, Sacristán B, et al. Barriers to HCV treatment in the era of triple therapy: a prospective multi-centred study in clinical practice. Liver Int 2015;35:401-8. DOI: 10.1111/liv.12536. [ Links ]
9. Deuffic-Burban S, Deltenre P, Buti M, et al. Predicted effects of treatment for HCV infection vary among European countries. Gastroenterology 2012;143:974-85. DOI: 10.1053/j.gastro.2012.05.054. [ Links ]
10. González-Grande R, Jiménez-Pérez M, González Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol 2016;22:1421-32. DOI: 10.3748/wjg.v22.i4.1421. [ Links ]
11. Asociación Española para el Estudio del Hígado. Guías AEEH/SEIMC de manejo de la Hepatitis C. 2016; (cited 2016 26th Ago). Available at: http://aeeh.es/wp-content/uploads/2016/06/Gu%C3%ADas_AEEH_SEIMC_VersionFinal.pdf. [ Links ]
12. European Association for the Study Liver (EASL). Recommendations on treatment of hepatitis C 2015. Clinical practice guidelines. (cited 2016 03th Oct). Available at: http://www.easl.eu/. Published 2015. [ Links ]
13. Spanish Ministry of Health, Social Policy and Equality. Plan Estratégico para el abordaje de la Hepatitis C (Internet). (cited 2016 23th Feb). Available at: http://www.msssi.gob.es. [ Links ]
14. Gentile I, Maraolo AE, Niola M, et al. Limiting the access to direct-acting antivirals against HCV: an ethical dilemma. Expert Rev Gastroenterol Hepatol 2016;10:1227-34. DOI: 10.1080/17474124.2016.1234375. [ Links ]
15. Calvaruso V, Craxi A. Why do I treat my patients with mild hepatitis C? Liver Int 2016;36(Suppl. S1):7-12. DOI: 10.1111/liv.13011. [ Links ]
16. Hézode C. Why I do not treat patients for mild disease. Liver Int 2016;36(Suppl. S1):13-20. DOI: 10.1111/liv.13019. [ Links ]
17. Obach D, Yazdanpanah Y, Esmat G, et al. How to optimize hepatitis C virus treatment impact on life years saved in resource-constrained countries. Hepatology 2015;62:31-9. DOI: 10.1002/hep.27691. [ Links ]
18. Adinolfi LE, Guerrera B. All-oral interferón-free treatments: The end of hepatitis C virus story, the dream and the reality. World J Hepatol 2015;7:2363-8. DOI: 10.4254/wjh.v7.i22.2363. [ Links ]
19. Buti M, Domínguez-Hernández R, Oyagüez I, et al. Cost-effectiveness analysis of ledipasvir/sofosbuvir in patients with chronic hepatitis C: Treatment of patients with absence or mild fibrosis compared to patients with advanced fibrosis. J Viral Hepat 2017. DOI: 10.1111/jvh.12704. DOI: 10.1111/jvh.12704. [ Links ]
20. Spanish Ministry of Health, Social Policy and Equality. Institute for Health Information. Press releases, 2016 (Internet) (cited 2016 23th Feb). Available at: http://www.msssi.gob.es/gabinete/notasPrensa.do?id=3909. [ Links ]
21. Turnes J, Domínguez-Hernández R, Casado MA. Cost-effectiveness analysis of two treatment strategies for chronic hepatitis C: before and after access to direct-acting antiviral in Spain. Gastroenterol Hepatol 2017;40:433-46. DOI: 10.1016/j.gastrohep.2017.05.004. [ Links ]
22. Buti M, San Miguel R, Brosa M, et al. Estimating the impact of hepatitis C virus therapy on future liver-related morbidity, mortality and costs related to chronic hepatitis C. J Hepatol 2005;42:639-45. DOI: 10.1016/j.jhep.2004.12.031. [ Links ]
23. Puente Á, Cabezas J, López Arias MJ, et al. Influence of sustained viral response on the regression of fibrosis and portal hypertension in cirrhotic HCV patients treated with antiviral triple therapy. Rev Esp Enferm Dig 2017;109:17-25. [ Links ]
24. Chahal HS, Marseille EA, Tice JA, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med 2016;176:65-73. DOI: 10.1001/jamainternmed.2015.6011. [ Links ]
25. Ferrante SA, Chhatwal J, Brass CA, et al. Boceprevir for previously untreated patients with chronic hepatitis C genotype 1 infection: a US-based cost-effectiveness modeling study. BMC Infect Dis 2013;13:190. DOI: 10.1186/1471-2334-13-190. [ Links ]
26. Maylin S, Martinot-Peignoux M, Moucari R, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology 2008;135:8219. [ Links ]
27. Saab S, Hunt DR, Stone MA, et al. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl 2010;16:748-59. DOI: 10.1002/lt.22072. [ Links ]
28. San Miguel R, Gimeno-Ballester G, Blázquez A, et al. Cost-effectiveness analysis of sofosbuvir-based regimens for chronic hepatitis C. Gut 2015;64:1277-88. DOI: 10.1136/gutjnl-2014-307772. [ Links ]
29. Younossi ZM, Park H, Saab S, et al. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther 2015;41:544-63. DOI: 10.1111/apt.13081. [ Links ]
30. Martin NK, Vickerman P, Dore GJ, et al. Priorization of HCV treatment in the direct-acting antiviral era: An economic evaluation. J Hepatol 2016;65:17-25. DOI: 10.1016/j.jhep.2016.02.007. [ Links ]
31. Spanish General Council of Official Colleges of Pharmacists - Bot PLUS 2.0 (cited 2016 23th May). Available at: https://botplusweb.portalfarma.com/. [ Links ]
32. Ministry of Finance and Public Administration. Update on the stability program 2016-2019 (internet). (cited 2016 23th Feb) Available at: http://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/docs/plan_estrategico_hepatitis_C.pdf. [ Links ]
33. Buti M, Gros B, Oyagüez I, et al. Cost-utility analysis of triple therapy with telaprevir in treatment-naïve hepatitis C patients. Farm Hosp 2014;38(5):418-29. [ Links ]
34. Drummond MF, Sculpher MJ, Torrance GW, et al., editors. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005. [ Links ]
35. López-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ 2010;11:513-20. DOI: 10.1007/s10198-010-0244-4. [ Links ]
36. Catalá F, García-Altés A. Algunas consideraciones metodológicas en la evaluación económica de intervenciones preventivas: un debate abierto. PharmacoEconomics - Spanish Research Articles 2008;5:61-5. DOI: 10.1007/BF03320838. [ Links ]
37. Van Nuys K, Brookmeyer R, Chou JW, et al. Broad hepatitis C treatment scenarios return substantial health gains, but capacity is a concern. Health Aff (Millwood) 2015;34:1666-74. DOI: 10.1377/hlthaff.2014.1193. [ Links ]
38. Sacristán JA, Oliva J, del Llano J, et al. What is an efficient health technology in Spain Gac Sanit 2002;16:334-43. [ Links ]
39. Vallejo-Torres L, García-Lorenzo B, Castilla I, et al. Valor Monetario de un Año de Vida Ajustado por Calidad: Estimación empírica del coste de oportunidad en el Sistema Nacional de Salud. Ministerio de Sanidad, Servicios Sociales e Igualdad. Informes de Evaluación de Tecnologías Sanitarias. Servicio de Evaluación del Servicio Canario de la Salud; 2015. Available at: http://www3.gobiernodecanarias.org/sanidad/scs/content/3382aaa2-cb58-11e5-a9c5-a398589805dc/SESCS%202015_Umbral%20C.O.%20AVAC.pdf. [ Links ]
40. Nuño Solinís R, Arratibel Ugarte P, et al. Value of Treating All Stages of Chronic Hepatitis C: A Comprehensive Review of Clinical and Economic Evidence. Infect Dis Ther 2016;5: 491-508. DOI: 10.1007/s40121-016-0134-x. [ Links ]
41. Arganda C. El gasto farmacéutico crece por debajo del PIB y la industria no devolverá. DiarioFarma, Madrid: 2017, enero 23. (internet). Available at: https://www.diariofarma.com/2017/01/23/gasto-farmaceutico-crece-debajo-del-pib-la-industria-no-devolvera. [ Links ]
42. Porter ME. What is value in health care? N Engl J Med 2010;363:2477-81. [ Links ]
43. McMahon LF Jr, Chopra V. Health care cost and value: the way forward. JAMA 2012;307:671-2. DOI: 10.1001/jama.2012.136. [ Links ]
44. Vernaz N, Girardin F, Goossens N, et al. Drug Pricing Evolution in Hepatitis C. PLoS ONE 2016;11:e0157098. DOI: 10.1371/journal.pone.0157098. [ Links ]
45. Belli LS, Berenguer M, Cortesi PA, et al. European Liver and Intestine Association (ELITA). Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol 2016;65:524-31. DOI: 10.1016/j.jhep.2016.05.010. [ Links ]
46. Jena AB, Stevens W, Gonzalez YS, et al. The wider public health value of HCV treatment accrued by liver transplant recipients. Am J Manag Care 2016;22:SP212-9. [ Links ]
47. Sociedad Española de Trasplante Hepático (SETH). Registro Español de Trasplante Hepático. Memoria de resultados 2015. Memoria trasplante hepático 2015. (cited 2016 10th Nov). Available at: http://www.sethepatico.org. [ Links ]
48. Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making 2000;20:332-42. DOI: 10.1177/0272989X0002000310. [ Links ]
49. Tang L, Marcell L, Kottilil S. Systemic manifestations of hepatitis C infection. Infect Agent Cancer 2016;11:29. DOI: 10.1186/s13027-016-0076-7. [ Links ]
50. Younossi Z, Brown A, Buti M, et al. Impact of eradicating hepatitis C virus on the work productivity of chronic hepatitis C (CH-C) patients: an economic model from five European countries. J Viral Hepat 2016;23:217-26. DOI: 10.1111/jvh.12483. [ Links ]
51. Oliva-Moreno J, Peña-Longobardo LM, Alonso S, et al. Labour productivity losses caused by premature death associated with hepatitis C in Spain. Eur J Gastroenterol Hepatol 2015;27:631-7. DOI: 10.1097/MEG.0000000000000336. [ Links ]
![]() Correspondence:
Correspondence:
Juan Turnes.
Department of Gastroenterology and Hepatology.
Complejo Hospitalario Universitario Pontevedra.
Rúa Montecelo, s/n.
36071 Pontevedra
e-mail: jturnesv@gmail.com
Received: 25-05-2017
Accepted: 04-09-2017