Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.1 Madrid Jan. 2018
https://dx.doi.org/10.17235/reed.2017.5210/2017
ORIGINAL PAPERS
Effectiveness of direct-acting antiviral therapy in patients with a HCV/HIV coinfection. A multicenter cohort study
1Hospital Universitario de Guadalajara. Guadalajara. España
2Hospital Virgen de la Salud de Toledo. Toledo. España
3Hospital General de Ciudad Real. Ciudad Real. España
4Hospital General Virgen de la Luz. Cuenca. España
5Hospital General Universitario de Albacete. Albacete. España
6Hospital General La Mancha Centro. Alcázar de San Juan, Ciudad Real. España
7Hospital Nuestra Señora del Prado. Talavera de la Reina, Toledo. España
8Hospital General de Villarrobledo. Villarrobledo, Albacete. España
9Grupo de Estudio de Castilla-La Mancha de Enfermedades Infecciosas (GECMEI). España
INTRODUCTION
Hepatitis C virus (HCV) infection is one of the most common comorbidities in patients infected with the human immunodeficiency virus (HIV) 1. Among the 185 million chronically infected individuals with HCV worldwide, approximately five million are also infected with HIV 2. However, the seroprevalence of HCV in HIV infected patients has gradually decreased in Spain. Recent data from Spanish cohorts show seroprevalence rates of 37.7% for anti-HCV antibodies and active disease rates of 22.1% (with detectable HCV RNA) among coinfected individuals 3. Factors that have contributed to this decrease include a dramatic reduction in parenteral substance consumption, which is the main route for HIV transmission, which started in 1997 and the implementation of damage reduction programs in Spain.
It is well known that concomitant infection with HIV modifies the natural history of HCV infection, resulting in an accelerated development of fibrosis and a higher risk of end-stage liver disease 4. Observational cohort studies have shown that HCV clearing is associated with lower morbidity and mortality rates from both liver and non-liver disease in these patients 5. Reaching a sustained viral response (SVR) results in a significant reduction in fibrosis progression, hepatocarcinoma development and death from hepatopathy, not only in patients with advanced fibrosis but also in patients with moderate fibrosis 6. Historically, the standard management of HCV infection was based on dual therapies including pegylated interferon and ribavirin, which were beneficial for most mono-infected patients. SVR rates were substantially lower in coinfected subjects 7, who also experienced a high rate of side effects 8. Subsequently, the advent of first-generation protease inhibitors (telaprevir, boceprevir) in 2011, which were used in combination with pegylated interferon and ribavirin, resulted in similar response rates for both groups. However, their high cost, unfavorable toxicity profile and numerous interactions with antiretroviral drugs (ARDs) precluded their large-scale use.
The development of direct-acting antivirals (DAAs) has led to a huge improvement in the prognosis of patients with chronic HCV infection 9. These oral, interferon-free approaches have proven superior to all prior schemes since their introduction in 2014 10. The efficacy of DAAs has been studied in clinical trials with both monoinfected and coinfected subjects 11) (12) (13) (14. However, evidence of the effectiveness of these therapies in Spain is insufficient in prospective, multicenter cohorts.
The goal of this study was to analyze the real-life effectiveness and toxicity of DAA therapy in patients coinfected with HIV and HCV and to identify variables associated with unfavorable outcome.
PATIENTS AND METHODS
Study design and population
An ambispective, multicenter, cohort study was performed with 229 patients with HCV/HIV coinfection who were under active follow-up at HIV clinics within eight healthcare institutions in the Castilla-La Mancha Autonomous Community. The study was conducted from September 1st 2014 to December 31st 2016. Adult patients (above 18 years of age) with HCV/HIV coinfection under DAA therapy for HCV were enrolled into the study. Patients undergoing regimens that included pegylated interferon were excluded. All treated patients with a positive HCV serology and ribonucleic acid (RNA) were analyzed regardless of the viral load. HCV therapy-naïve cases and patients that failed to respond to prior treatment with either interferon or interferon plus boceprevir, telaprevir or simeprevir were included. Antiretroviral therapy (ART) regimens and DAAs were selected by the researchers according to availability, toxicity or interaction profile. Patients not receiving ART were required to have CD4 T-cell counts above 350 cells per mm3 (cells/mm3). Patients infected with HCV genotypes 1, 2, 3 and 4 were included in the study.
Data collection
All patients under the care of the collaborating HIV units were recorded in an internal database within each center. Patients included in the study were identified from these databases and primary variable (efficacy and toxicity) values were prospectively collected at each clinic by filling in a form. Many of the epidemiological, biological, clinical, laboratory, radiographic, histological and elastographic data were retrospectively collected by careful review of medical records.
The study was carried out in accordance with good clinical practice guidelines and with the approval of the Ethics Committee at the Hospital Universitario de Guadalajara.
Definitions and study variables
Variables collected at baseline included age, sex, transmission route, HIV-related Center for Disease Control and Prevention (CDC) classification, HCV load prior to DAA onset, HCV genotype, HIV load prior to DAA onset, absolute and relative CD4 T-cell counts prior to DAA onset, concomitant antiretroviral therapy, esophageal varices status, splenomegaly, portal hypertension, HBV surface antigen, interleukin B28 (IL28B) polymorphism, hepatic comorbidities, ascites, encephalopathy, history of enolism and history of prior HCV therapies.
HCV VL before and after treatment and the sustained viral response at 12 weeks after therapy completion were determined. The extent of liver fibrosis (in kilopascals) was assessed via vibration-controlled transient elastography (FibroScan(r) 402) at treatment onset. The laboratory variables included creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gammaglutamyl transpeptidase (GGT), platelets, total bilirubin, INR and albumin. The Child-Pugh stage before DAA therapy onset was analyzed in the cirrhotic population.
Liver cirrhosis was defined as any of the following: a liver biopsy consistent with cirrhosis at any time before or during screening, or liver stiffness higher than 12.5 kPa according to the pre-treatment elastogram. The intake of at least 50 g of alcohol a day in females or 70 g daily in males was considered as alcoholism as well as alcohol addiction diagnosed by a psychiatry unit or followed up by an alcohol rehabilitation unit anytime during the patient's history. Laboratory and clinical studies were performed for all patients before starting therapy, after completing therapy and 12 weeks after therapy. SVR was defined as a negative HCV-RNA detection test 12 weeks after DAA therapy was completed. Virologic failure was defined as at least a 1 log10 increase in HCV-RNA from the lowest point, quantifiable HCV-RNA when previously undetectable during therapy (breakthrough) or quantifiable HCV-RNA after completion of the treatment (relapse). Therapy failure was defined as any cause resulting in the absence of SVR 12 weeks after treatment completion, including virologic failure, loss to follow-up, death from all causes, treatment discontinuation due to toxicity, pregnancy, poor compliance and treatment abandonment.
Primary and secondary endpoints
The primary endpoint was real-life efficacy (effectiveness), defined as SVR. An intent-to-treat analysis was performed where loss to follow-up, treatment abandonment and toxicity were considered as failures. Secondary endpoints included a toxicity analysis (adverse events and therapy dropout causes) and the analysis of factors associated with the absence of SVR.
Statistical analysis
Percentage and interquartile range (IQR) were used for categorical and quantitative variables. The 2 test and Fisher's exact test when necessary were used for the comparison of categorical variables, and the Student's t-test and ANOVA were used for the comparison of quantitative variables. Odds ratios and the corresponding 95% confidence intervals were used as a measure of risk. Univariate and multivariate logistic regression was used for the study of independent unfavorable response risk factors; the binary dependent variable was SVR status. The SPSS 20.0 statistical package was used for data analysis. All tests were two-tailed and statistical significance was set at p < 0.05.
RESULTS
A total of 229 patients with a median age of 49.6 years (IQR 46.7-52.8) were included in the study; 83% (190) were male. Table 1 shows the clinical, laboratory and epidemiological characteristics of the patients. Most patients had genotype 1, and genotypes 3 and 4 were less common. The cohort represented a population with advanced liver disease, 50% had cirrhosis (20% Child B or C) and 25% had enolism. In addition, 65% of cases had more than 800,000 copies/ml of HCV VL and the IL28b gene polymorphism was an unfavorable factor in the majority of cases (65%). Baseline liver fibrosis data was collected in all patients except for 14 cases. A liver biopsy was available in three of these cases and the rest had either clinical signs of cirrhosis, had previously received interferon and ribavirin or had obesity-related technical difficulties.
Table 1 Baseline epidemiological, clinical and laboratory characteristics of the study population


IQR: Interquartile range; HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: Gammaglutamyl transpeptidase; INR: International normalized ratio; HBV: Hepatitis B virus; CDC: Centers for Disease Control and Prevention; kPa: Kilopascals; MSM: Males who have sex with males. aMeasured in patients with baseline liver cirrhosis. bPortal hypertension diagnosed via upper GI endoscopy or an imaging study. cLiver rigidity determined via transient elastography. dDrinking habit sometime during the patient's history.
Table 2 shows the antiretroviral therapy regimens used by researchers. The most widely used regimens included tenofovir disoproxil fumarate and integrase inhibitors (mainly raltegravir). Sustained viral response was reached by 91.7% of patients (intention-to-treat analysis) and 95.6% had undetectable HCV VL levels after treatment completion. No variables were identified that predicted virologic or therapeutic failure with regard to SVR (Fig. 1). The univariate analysis found no association between SVR and other variables such as platelet count, albumin, or Child-Pugh classification in cirrhotic patients. DAA therapy strategies are summarized in figure 2 and 48% of regimens also included ribavirin. Treatment duration was variable: 65.9% completed 12-week regimens, 30.1% completed 24-week schemes and only 3.9% received shorter 8-week dose schedules.
Table 2 Antiretroviral treatment regimens used during DAA administration

NRTI: Nucleoside reverse-transcriptase inhibitor; NNRTI: Non-nucleoside reverse-transcriptase inhibitor; HIV: Human immunodeficiency virus; TDF: Tenofovir disoproxil fumarate; ABC: Abacavir.

Fig. 1 Univariate analysis: variables that predict an unfavorable outcome. The graph illustrates the main variables included in the univariate analysis. No variable was shown to predict an unfavorable outcome (OR: Odds ratio; CI: Confidence interval; HIV: Human immunodeficiency virus; TDF: Tenofovir disoproxil fumarate; ABC: Abacavir; IFN: Pegylated interferon; Rib: Ribavirin).
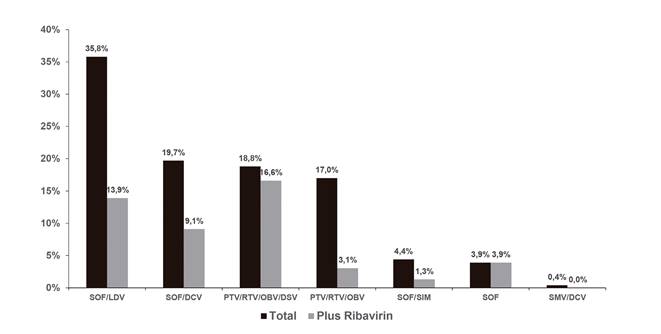
Fig. 2 Direct-acting antiviral strategies. The figure shows the direct-acting antiviral strategies that were used and the percentage of patients receiving ribavirin within each group (SMV: Simprevir; LDV: Ledipasvir; SOF: Sofosbuvir; DCV: Daclatasvir; OBV: Ombitasvir; PTV: Paritaprevir; DSV: Dasabuvir; RTV: Ritonavir).
SVR according to the various subgroups is summarized in figure 3. No statistically significant differences were found with regard to DAA therapy strategies. There were 19 therapy failures and these included ten relapses (distributed among all groups except for paritaprevir/ritonavir/ombitasvir), one breakthrough (in the sofosbuvir/ledipasvir group, relating to poor patient adherence) and three dropouts due to toxicity (two in the sofosbuvir/simeprevir group and one in the sofosbuvir/daclatasvir group). The relapsed patients had the same genotype after a repeat test, although reinfection cannot be definitely excluded as a phylogenetic study was not performed. In addition to the non-adherent patient, five patients dropped out due to causes unrelated to treatment: two died (one had sepsis and one had hepatorenal syndrome with hydropic decompensation after therapy onset) and three were lost to follow-up. Another individual died after reaching SVR and was diagnosed with advanced-stage lung adenocarcinoma. One further subject discontinued therapy due to toxicity without ever reaching SVR. Most reported adverse events included asthenia and gastrointestinal disorders. No baseline resistance tests were performed and only one test was carried out with the patient with breakthrough failure and no resistance-associated substitutions were found.
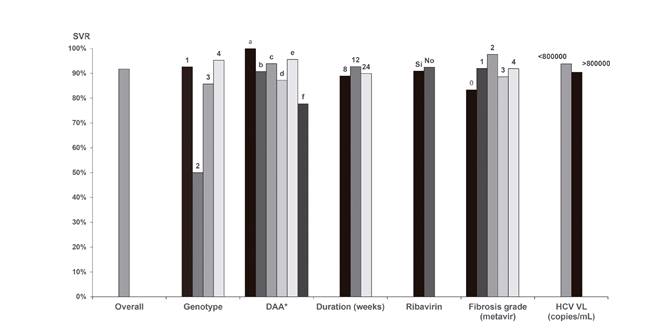
Fig. 3 Sustained viral response in the various subgroups. *Sustained viral response was recorded for the various DAA treatment groups. a) PTV/RTV/OBV; b) PTV/RTV/OBV/DSV; c) SOF/LDV; d) SOF/SMV; e) SOF/DCV; and f) SOF (DAA: Direct-acting antiviral; VL: Viral load; HCV: Hepatitis C virus; SMV: Simprevir; LDV: Ledipasvir; SOF: Sofosbuvir; DCV: Daclatasvir; OBV: Ombitasvir; PTV: Paritaprevir; DSV: Dasabuvir; RTV: Ritonavir).
A multivariate analysis was performed with logistic regression using backwards stepwise exclusion. SVR was the dependent variable whereas sex, age, cirrhosis, genotype 1 or 4 vs 3 and over 800,000 copies/ml of HCV VL were used as independent variables. No independent predictor of SVR was found.
DISCUSSION
Data from clinical trials of HCV treatment with DAAs in coinfected patients have shown similar effectiveness as compared to studies in mono-infected subjects 11) (12) (13) (14. Studies from mono-infected Spanish cohorts have been published 15) (16) (17) (18, however, there are few reports on the effectiveness of these therapies in prospective coinfected cohorts 19. This paper reports real-life SVR data obtained from a multicenter cohort. Patients in this cohort study had a median age of almost 50 years and were predominantly male. The proportion of patients with detectable HIV V (> 50 copies/ml) was < 10%. Strikingly, almost 45% of patients had fewer than 200 CD4 T-cells.
HCV/HIV coinfection is strongly associated with parenteral drug use. However, HCV epidemiology has changed over the years. There are recent reports of HCV microepidemics in populations that engage in risky sexual practices such as chemsex, particularly among men who have sex with men (MSM) 20) (21. This highlights the importance of ongoing health education for at risk groups in order to inform them of the steps to be taken to prevent virus transmission and to highlight the need for frequent serology tests. The latter remains a primary goal for the Plan estratégico para el abordaje de la hepatitis C en el Sistema Nacional de Salud, approved in Spain in 2015. Similarly, these data also warrant a proactive therapeutic approach towards patients, either with or without mild fibrosis (F0-F1).
HCV infection in this cohort mostly results from genotypes 1a and 4. This was also seen in other Spanish series and stems from the fact that these are the most common genotypes associated with parenteral drug use. Historically, this is the primary HCV transmission route in our study setting. Genotypes 2 and 1b were the least common and these genotypes have been linked to minor transmission routes (blood products transfusion). Data from our study are consistent with those of other Spanish series 22. This cohort also includes genotype-3 patients, the least common genotype with very limited evidence.
In contrast to specifically designed clinical trials in coinfected patients, our study had in excess of 45% of patients who met elastographic criteria for cirrhosis (Metavir F4) 13) (14. The vast majority of patients had a recent elastographic study of liver rigidity and no significant differences in SVR were observed between the cirrhosis and pre-cirrhosis groups or between the mild and moderate stiffness groups. No statistically significant differences were found among the various genotypes. In contrast to other studies 13) (14), relapse rates were similar when patients were stratified according to baseline HCV RNA; the cut-off was 800,000 copies/ml. Data from our cohort are similar to those reported by other observational studies in coinfected subjects, such as the French HEPAVIH cohort 23.
The prospective studies performed to analyze real-life DAA effectiveness have yielded similar results to those reported in clinical trials 24) (25. The therapy regimens recommended for these patients are essentially the same for mono-infected individuals, with the potential drug-drug interactions between ARDs and DAAs. There were differences with regard to effectiveness among the various DAA regimens in this study. The multivariate analysis attempted to identify variables that were potentially associated with an unfavorable outcome. No variable was independently associated with the prediction of a poor outcome in a statistically significant manner.
Our cohort's findings support the excellent results seen in clinical trials. DAA real-life effectiveness in patients with HCV/HIV coinfection is outstanding. Toxicity is exceptional, as are toxicity-related therapy discontinuations. This suggests that the high potency and safety shown by these drugs by far exceeds the predictors of poor outcome described in other studies.
Weaknesses and strengths
This study has the limitations associated with a cohort study and potential confounders that introduce a bias into the potential association of some variables with a sustained viral response. The small sample size precludes an adequate power to detect unfavorable outcome predictors and the use of a larger number of variables in the multivariate analysis. The strength of this study is its real-life, ambispective nature, with a large number of hospitals (some small centers) with an extraordinary efficacy of DAA therapy. The fact that no unfavorable outcome predictors were found suggests that, even if they existed, their significance is low or their impact is small with regard to curing HCV infection.
CONCLUSIONS
HCV treatment in patients with HIV infection reached very high sustained viral response rates. Real-life data and the clinical trials involved were excellent. Safety and tolerability are significant benefits of these drugs. No specific factors that predicted an unfavorable outcome were identified in this study.
ACKNOWLEDGEMENTS
The following authors, who are members of GECMEI (Grupo de Estudio de Castilla-La Mancha de Enfermedades Infecciosas), have also contributed to this paper: Irena Jiménez-Velasco, María Antonia Sepúlveda-Berrocal, Fernando Cuadra-García-Tenorio, Erika Yvette-Bencosme, Julio Antonio Gijón-Rodríguez and Fernando Marcos-Sánchez.
REFERENCES
1. Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: Evolving epidemiology and treatment paradigms. Clin Infect Dis 2012;55(Suppl 1):S33-42. DOI: 10.1093/cid/cis367 [ Links ]
2. Kohli A, Shaffer A, Sherman A, et al. Treatment of hepatitis C: A systematic review. JAMA 2014;312(6):631-40. DOI: 10.1001/jama.2014.7085 [ Links ]
3. Pérez Cachafeiro S, Del Amo J, Iribarren JA, et al. Decrease in serial prevalence of coinfection with hepatitis C virus among HIV-infected patients in Spain, 1997-2006. Clin Infect Dis 2009;48(10):1467-70. DOI: 10.1086/598333 [ Links ]
4. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. Clin Infect Dis 2001;33(4):562-9. DOI: 10.1086/321909 [ Links ]
5. Berenguer J, Rodríguez-Castellano E, Carrero A, et al. Eradication of HCV and non-liver-related non-AIDS-related events in HIV/HCV coinfection. Hepatology 2017;66(2):344-56. DOI: 10.1002/hep.29071 [ Links ]
6. Barreiro P, Labarga P, Martin-Carbonero L, et al. Sustained virological response following HCV therapy is associated with non-progression of liver fibrosis in HCV/HIV-coinfected patients. Antivir Ther 2006;11(7):869-77. [ Links ]
7. Hadigan C, Kottilil S. Hepatitis C virus infection and coinfection with human immunodeficiency virus: Challenges and advancements in management. JAMA 2011;306(3):294-301. DOI: 10.1001/jama.2011.975 [ Links ]
8. Kim AI, Dorn A, Bouajram R, et al. The treatment of chronic hepatitis C in HIV-infected patients: A meta-analysis. HIV Med 2007;8(5):312-21. DOI: 10.1111/j.1468-1293.2007.00476.x [ Links ]
9. Milazzo L, Lai A, Calvi E, et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med 2017;18(4):284-91. DOI: 10.1111/hiv.12429 [ Links ]
10. Menard A, Colson P, Catherine D, et al. First real life evidence of new direct-acting antivirals (DAA) in co-infected HIV HCV patients: Better than ever. Clin Infect Dis 2016;62(7):947-9. DOI: 10.1093/cid/civ1215 [ Links ]
11. Kwo P, Gane EJ, Peng C-Y, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology 2017;152(1):164-75.e4. DOI: 10.1053/j.gastro.2016.09.045 [ Links ]
12. Laufer NL, Rockstroh JK. Faldaprevir (BI 201335) for the treatment of hepatitis C in patients co-infected with HIV. Expert Rev Anti Infect Ther 2014;12(2):157-64. DOI: 10.1586/14787210.2014.868774 [ Links ]
13. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015;373(8):714-25. DOI: 10.1056/NEJMoa1503153 [ Links ]
14. Naggie S, Cooper C, Saag M, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015 20;373(8):705-13. DOI: 10.1056/NEJMoa1501315 [ Links ]
15. Alonso S, Riveiro-Barciela M, Fernández I, et al. Effectiveness and safety of sofosbuvir-based regimens plus an NS5A inhibitor for patients with HCV genotype 3 infection and cirrhosis. Results of a multicenter real-life cohort. J Viral Hepat 2017;24(4):304-11. DOI: 10.1111/jvh.12648 [ Links ]
16. Calleja JL, Crespo J, Rincon D, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J Hepatol 2017;66(6):1138-48. DOI: 10.1016/j.jhep.2017.01.028 [ Links ]
17. Crespo J, Calleja JL, Fernández I, et al. Real-world effectiveness and safety of oral combination antiviral therapy for hepatitis C virus genotype 4 infection. Clin Gastroenterol Hepatol 2017;15(6):945-9.e1. DOI: 10.1016/j.cgh.2017.02.020 [ Links ]
18. Marino Z, Pascasio-Acevedo JM, Gallego A, et al. High efficacy of sofosbuvir plus simeprevir in a large cohort of Spanish cirrhotic patients infected with genotypes 1 and 4. Liver Int 2017;37(12):1823-32. DOI: 10.1111/liv.13470 [ Links ]
19. Juanbeltz Zurbano R, Zozaya Urmeneta JM, Reparaz Padros J, et al. Effectiveness of second-generation direct-acting antivirals in chronic hepatitis C. An Sist Sanit Navar 2017;40(1):57-66. DOI: 10.23938/ASSN.0006 [ Links ]
20. Montoya-Ferrer A, Fierer DS, Álvarez-Álvarez B, et al. Acute hepatitis C outbreak among HIV-infected men, Madrid, Spain. Emerg Infect Dis 2011;17(8):1560-2. DOI: 10.3201/eid1708.110147 [ Links ]
21. Berenguer J, Rivero A, Jarrin I, et al. Human immunodeficiency virus/hepatitis C virus coinfection in Spain: Prevalence and patient characteristics. Open Forum Infect Dis 2016;3(2):ofw059. DOI: 10.1093/ofid/ofw059 [ Links ]
22. Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 2008;48(1):148-62. DOI: 10.1016/j.jhep.2007.07.033 [ Links ]
23. Piroth L, Wittkop L, Lacombe K, et al. Efficacy and safety of direct-acting antiviral regimens in HIV/HCV-co-infected patients - French ANRS CO13 HEPAVIH cohort. J Hepatol 2017;67(1):23-31. DOI: 10.1016/j.jhep.2017. 02.012 [ Links ]
24. Tapper EB, Bacon BR, Curry MP, et al. Real-world effectiveness for 12 weeks of ledipasvir-sofosbuvir for genotype 1 hepatitis C: The Trio Health study. J Viral Hepat 2017;24(1):22-7. DOI: 10.1111/jvh.12611 [ Links ]
25. Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology 2016;150(2):419-29. DOI: 10.1053/j.gastro.2015.10.013 [ Links ]
Received: August 24, 2017; Accepted: October 25, 2017











 texto em
texto em 


