My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 n.1 Madrid Jan. 2018
https://dx.doi.org/10.17235/reed.2017.5214/2017
LETTERS TO THE EDITOR
A new swallowable intragastric balloon (Elipse®). Coffee for everybody? The position of GETTEMO
1Hospital Universitario Dexeus. Barcelona. España
2Clínica Diagonal. Barcelona. España
3Hospital Universitario HM Sanchinarro. Madrid. España
Key words: Elipse; Intragastric balloon; Swallow balloon
Dear Editor,
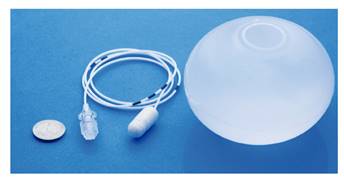
Recently, the Elipse® swallowable balloon with spontaneous evacuation (Allurion Technologies, Wellesley, Mass) has been incorporated into the clinic (Fig. 1). When rolled into a capsule, the balloon is ingested and filled under radiological control via a thin catheter. Over a period of 16 weeks, the balloon degrades, weakens and opens, allowing the balloon to empty and to be eliminated naturally. The first observational studies report an adequate efficacy and safety 1) (2). In general, its indications and contraindications overlap with those previously described for the other balloons 1) (2) (3. Unlike other procedures, this technique does not necessarily require endoscopy or sedation or anesthesia for implantation or extraction. However, in order to avoid any unnecessary risks and to rule out any contraindications, prior imaging, either radiological or ideally endoscopic technique may be required in order maximize safety.
This procedure must be carried out by physicians with an extensive gastrointestinal knowledge, previous experience in balloons and a basic knowledge in radiology. Any complication must be resolved endoscopically, quickly and effectively. Therefore, this tool can be implanted by a bariatric endoscopist or under the supervision of a bariatric endoscopist. The possibility of an incomplete deflation with migration may increase the risk of intestinal obstructions, which may require surgical extraction when detected late 4.
In summary, GETTEMO supports innovations in the endoscopic treatment of bariatric patients such as this new gastric balloon, when performed with a suitable protocol and by a multidisciplinary team. In most cases, a prior endoscopy is required in order to rule out and effectively resolve complications and to ensure the maximum safety. The balloon must be implanted (or supervised) by a bariatric endoscopist and access to an endoscopic emergency department is required.
Conflicts of interest: The authors have no conflict of interests to declare with regard to this letter. This letter has been previously approved by the Spanish Working Group for the Endoscopic Treatment of Metabolism and Obesity (GETTEMO) of the SEED and the SEPD. Thus, it is written representing the opinion of this Group.
BIBLIOGRAFÍA
1. Machytka E, Gaur S, Chuttani R, et al. Elipse, the first procedureless gastric balloon for weight loss: a prospective, observational, open-label, multicenter study. Endoscopy 2017;49(2):154-160. DOI: 10.1055/s-0042-119296 [ Links ]
2. Raftopoulos I, Giannakou A. The Elipse Balloon, a swallowable gastric balloon for weight loss not requiring sedation, anesthesia or endoscopy: a pilot study with 12-month outcomes. Surg Obes Relat Dis 2017;13(7):1174-1182. DOI: 10.1016/j.soard.2017.02.016 [ Links ]
3. Genco A, López-Nava G, Wahlen C, et al. Multi-centre European experience with intragastric balloon in overweight populations: 13 years of experience. Obes Surg 2013;23(4):515-21. DOI: 10.1007/s11695-012-0829-3 [ Links ]
4. Espinet Coll E, Nebreda J, López-Nava G, et al. Estudio multicéntrico de seguridad en el tratamiento endoscópico de la obesidad. Rev Esp Enferm Dig 2017;109(5):350-7. DOI: 10.17235/reed.2017.4499/2016 [ Links ]











 text in
text in