INTRODUCTION
Nowadays, small bowel capsule endoscopy (SBCE) is considered as the first-line procedure for the visualization of the small bowel (SB) 1) (2) (3. Since its introduction, SBCE has evolved to become a widely accepted tool. It has revolutionized the approach for the investigation and management of SB diseases, as it is a minimally invasive technique that directly visualizes the mucosal surface usually inaccessible via conventional endoscopy 4) (5) (6) (7. Designed primarily to provide diagnostic imaging of the SB, SBCE has been used predominantly for obscure gastrointestinal bleeding (OGIB) and suspected Crohn's disease 8) (9. However, due to its excellent safety profile, other indications have been established during recent years, including the assessment of celiac disease, investigation of SB tumors and the surveillance of hereditary polyposis syndromes 10) (11. Unlike OGIB, where SB examination is typically indicated when no source of bleeding is identified via conventional endoscopy 12) (13, prior conventional endoscopy (gastroscopy and ileocolonoscopy) is not always mandatory when a SBCE is indicated. However, SBCE provides an opportunity to examine other areas of the gastrointestinal (GI) tract such as the esophagus, stomach, duodenum or colon 14) (15. Furthermore, it may detect lesions in proximal and distal segments of the GI tract that could have been overlooked by conventional endoscopy 16) (17. It is well known that both upper and lower GI endoscopic procedures have false negatives 18) (19) (20. However, the incidence and impact of these lesions on patient management has not been well documented. As a result, it is not clear whether all images of a video capsule procedure should be reviewed. The aim of the current study was to evaluate the incidence of gastroduodenal lesions (GDL) in patients that underwent SBCE and its impact on patient management.
PATIENTS AND METHODS
This study is a retrospective analysis of data from 2,217 consecutive SBCE performed at a single tertiary-care center (Complejo Hospitalario de Navarra, Spain) between January 2008 and February 2016. All patients with incomplete data were excluded from the study. The variables analyzed included patient demographics, procedure indications, presence and type of GDL during SBCE, performance of gastroscopy before and after SBCE, patient diagnosis and management before and after SBCE and patient outcome.
Definitions
Gastroscopy pre-SBCE: only those gastroscopies performed < 30 months before a SBCE procedure were considered.
Additional findings: refer to GDL different to those detected by a previous gastroscopy.
New findings: GDL detected in patients with no previous findings from the gastroscopy (i.e., negative gastroscopy).
Clinical impact: defined as the proportion of patients with changes in their pre-SBCE procedure diagnosis.
Therapeutic impact: defined as the proportion of patients with changes in their pre-SBCE procedure treatment.
Capsule endoscopy procedure
All SBCE examinations were performed using the PillCam(r)SB2 (January 2008- November 2012) and the PillCam(r)SB3 (December 2012-February 2016) manufactured by Given Imaging, Yoqneam, Israel. Patients underwent SBCE without any bowel preparation or prokinetics, with clear liquids the afternoon before and after fasting for eight hours. Up to 76.7% of the explorations were performed in an outpatient setting. Following administration of the capsule, patients were allowed to eat a light breakfast after three hours and a light meal after five hours. At the end of the recording period, patients returned to the endoscopy unit where the data recorder was removed and images were downloaded. SBCE recordings were reviewed by four experienced readers 21 (defined as readers with an experience of at least 200 SBCE explorations, 2-3 capsule readings per week and a professional interest in the procedure) at 12 frames per second using the Rapid(r) Reader software.
Statistical analysis
Statistical analysis was performed using the 15.0 version of the SPSS software (IBM Corporation, New York, USA). Normally distributed quantitative data are presented as the mean, standard deviation (SD) and range within the given values. Non-normally distributed quantitative data are presented as a median with the corresponding interquartile range. Qualitative variables are presented as simple proportions. The Chi-squared test was used for the qualitative data comparison and p values less than 0.05 were considered as statistically significant.
RESULTS
Two thousands and two hundred twenty-four SBCE procedures were performed during the study period. Seven capsule explorations were excluded from the analysis due to missing data. Therefore, 2,217 procedures were finally included in the analysis. One thousand and seventy patients were male (48.2%) and the mean age was 56.1 ± 19.5 years (range: 12-93). Patients were referred for SBCE due to OGIB in 1,160 cases (52.3%), known or suspected Crohn´s disease in 406 cases (18.3%), abdominal pain in 184 cases (8.3%), chronic diarrhea in 144 cases (6.5%) and other indications in 323 cases (14.6%). SB abnormalities were noted in 1,301 patients (58.6%) and these included erosions or ulcers in 36.2% (n = 471) of cases, angiodysplasias in 32.0% (n = 416), inflammatory bowel mucosa in 16.3% (n = 212), tumors in 5.0% (n = 65), active bleeding in 4.6% (n = 60) and other lesions in 5.9% (n = 77) of cases. The SB was not explored in 30 patients (1.4%) because the capsule was retained in the stomach and did not pass through the pylorus after the batteries expired.
Gastroduodenal lesions
SBCE detected 696 GDL in 566 patients, 285 (50.3%) patients had only gastric lesions (GL), 151 (26.7%) only duodenal lesions (DL) and 130 (23.0%) presented simultaneous gastric and duodenal lesions. Findings were identified simultaneously in the SB and stomach and/or duodenum in 370 of 566 patients (65.4%) undergoing SBCE. Up to 488 patients (86.2%) had a previous upper GI endoscopy, 463 (81.8%) were performed < 30 months and 25 (18.2%) > 30 months before the capsule procedure. The mean number of gastroscopies was 1.29 ± 1.1 1) (2) (3) (4) (5) (6) (7) (8) (9) (10 and the mean time between upper GI endoscopy and SBCE was 237.6 ± 527.4 days (0-3650).
Gastric lesions
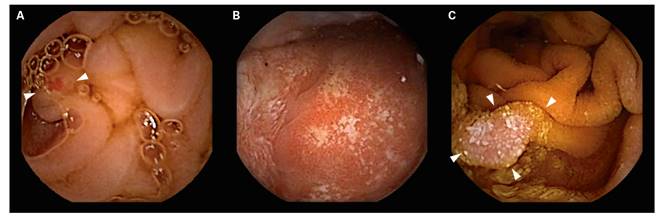
SBCE detected GL (Table 1) in 415 patients (18.7%). The most frequent locations for these lesions were the antrum in 42.9% (n = 178) of cases, the gastric body in 10.6% (n = 44), diffuse gastric affectation in 5.1% (n = 21), the cardial region in 0.7% (n = 3) and the gastric fundus in 0.7% (n = 3). The gastric location was not determined in 40% of cases (n = 166). Taking into account only those patients with an upper endoscopy < 30 months before SBCE (n = 341; 82.2%), GL were detected in 257 (75.4%) cases. These were new findings that were different to those detected by the previous gastroscopy (i.e., additional findings) in 98 patients (38.1%) and new findings in 159 patients (61.9%). Gastric lesions were also found in 74 patients with no previous gastroscopy (17.8%), these included gastric erosions in 48.6% (n = 36) of cases, findings suggestive of gastritis in 20.3% (n = 15), gastric ulcers in 12.2% (n = 9), polyps in 6.7% (n = 5), vascular lesions in 6.7% (n = 5), active bleeding in 4.1% (n = 3) and gastric tumors in 1.4% (n = 1) of cases. The types of lesions detected in the stomach during SBCE are shown in figure 1.
Table 1 Type of gastric findings during small bowel capsule endoscopy (SBCE)

PHG: portal hypertensive gastropathy; IM: intestinal metaplasia; GAVE: gastric vascular ectasia.
Duodenal lesions
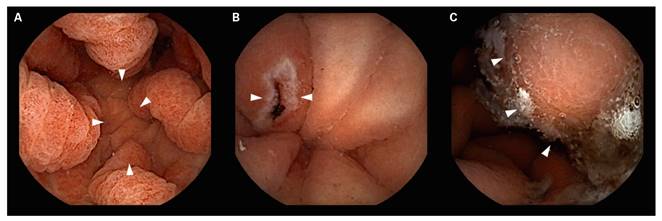
SBCE detected DL (Table 2) in 281 patients (12.7%). Taking into account only those patients with an upper endoscopy 30 months before the SBCE (n = 220; 78.3%), DL were detected 190 (86.4%) cases. These were new findings different to those detected by the previous gastroscopy (i.e., additional findings) in 32 patients (16.8%) and new findings in 158 patients (83.2%). DL were also found in 61 patients (21.7%) with no previous gastroscopy and these included duodenal erosions in 39.4% (n = 24) of cases, duodenal erythema in 26.2% (n = 16), ulcers in 14.8% (n = 9), vascular lesions in 8.2% (n = 5), Brunner´s gland hyperplasia in 3.3% (n = 2), active bleeding in 4.9% (n = 3), findings suggestive of duodenal atrophy in 1.6% (n = 1) and polyps in 1.6% (n = 1) of cases. The types of lesions detected in the duodenum during SBCE are shown in figure 2.
Clinical and therapeutic impact
GL and DL during SBCE led to a diagnostic change in 331 and 251 patients respectively, resulting in an overall clinical impact of 26.2%. The frequency of diagnostic changes was higher among patients with no previous gastroscopy (100% versus 80.5%, p < 0.05). Although, 463 (81.8%) of all patients with GDL had a previous gastroscopy, a second upper endoscopy was required in 104 patients (18.4%). On the other hand, due to the presence of GDL during CE, 343 (60.6%) patients underwent a change in their initial therapeutic strategy, resulting in an overall therapeutic impact of 15.5%. The frequency of therapeutic changes was significantly higher among those patients who had a previous gastroscopy (67.2% versus 48.2%, p < 0.05). Pharmacological therapy was the treatment of choice in 292 patients (85.1%), followed by therapeutic endoscopy in 44 patients (12.8%), surgery in 4 patients (1.2%) and a gluten-free diet in 3 patients with discrepant celiac-specific serology and duodenal histology (0.9%). Iron supplements (n = 167) in the pharmacological group and Argon Beam (n = 30) for vascular lesions (angiodysplasias) in the endoscopic group were the most common treatment changes found in the study after SBCE. Among patients taking iron supplements, oral iron supplements was first indicated in 82.6% of patients (n = 138) after SBCE findings, while oral supplements were changed for endovenous iron supplements in the remaining cases (n = 29). The study results are summarized in figure 3.
DISCUSSION
SBCE was developed to examine the SB in a simple and non-invasive way 4) (5) (6 (7, demonstrating its ability to visualize SB lesions in various studies 1) (2) (3. In 2001, SBCE was accepted for the study of the small intestine in patients with OGIB when no source of bleeding was identified after a negative conventional endoscopy (gastroscopy and colonoscopy) 8) (9. However, due to its excellent safety profile and the fact that it is well accepted by patients and physicians, other indications have been established in the last few years 10. Nowadays prior conventional endoscopy (gastroscopy and ileocolonoscopy) is no longer mandatory as it was over a decade ago due to the emerging indications for SBCE 11. However, SBCE allows the examination of the small intestine and other areas of the GI tract that are reached by the SBCE until the battery life expires 14) (15. In fact, some studies have reported the ability of SBCE to detect lesions outside the small intestine and sometimes within the reach of a conventional gastroscopy, meaning that they could have been missed during prior examinations 16) (17. Therefore, the unanswered question and the main objective of the study were to determine whether we should review all the images of the SBCE videos. The incidence of GDL detected during SBCE varies among different studies, ranging from less than 5% to as high as 20% 22) (23) (24. OGIB was the principal indication for performing SBCE in all of these studies. In contrast, although OGIB is still the main indication in the present study (52.3%), other indications such as Crohn´s or celiac disease have also been included. Up to 31.4% of non-SB lesions (i.e., upper GI) were identified. In contrast to other studies, the number of GDL in our study may be unusually high. This may be explained by the fact that we consider that all findings detected should be always collected in the final report. This could be important when patients are referred from other medical services or medical centers, in the outpatient setting and for those patients who undergo a capsule endoscopy with incomplete medical information. These non-small bowel findings were commonly found in the stomach (50.3%) and duodenum (26.7%), although simultaneous gastric and duodenal lesions were identified in 23.0% of cases. Typically, GL were described in the gastric body or antrum (58.6%), underlining the limitations of the SBCE for examining other segments such as the gastric fundus or cardia region 25) (26. The duodenum is also usually explored during SBCE and the bulb is the most frequent location for DL. However, a complete examination of the duodenum is not always feasible, as SBCE transit time in this segment is very short 27) (28) (29. To date, it is not clear why these lesions revealed by SBCE are missed during the initial upper endoscopy. Although some possible explanations may be related to lesions characteristics (size of the lesion and/or unusual location) and the endoscopic procedures (quality of the exploration, complete examination rates or endoscopist experience) 23) (30. Indeed, there are a few studies that have reported the value of SBCE for the detection of missed lesions, not only in the small intestine but also in the upper and lower GI tract 22) (23) (24) (31) (32. However, they describe few limitations related to capsule characteristics that may complicate gastric visibility including: a) the absence of gastric distension; b) the presence of gastric residues despite overnight fasting; and c) gastric time release (time from ingestion to passage through the pylorus) 23. Despite these limitations and as we have shown previously, the stomach and duodenum were adequately visualized by SBCE in the majority of cases. This allowed the identification of a significant proportion of new lesions in the upper GI tract (31.4%) and led to an improved diagnostic yield in 26.2% of the overall patients. However, many of these lesions were not histologically confirmed and upper GI endoscopy was indicated after SBCE findings in only 18.4% of cases, which made the interpretation of the results difficult. Nonetheless, they led to changes in the management of 15.5% of the patients undergoing SBCE. This value is even higher in those patients with no previous upper GI endoscopy. Therefore, we may consider SBCE as a complementary technique to gastroscopy and a careful review of the gastroduodenal images should be recommended, even more so when an initial gastroscopy was not performed.
The current study faced the following limitations: a) a retrospective study design with a simple descriptive analysis without a comparison or profound investigation of the SBCE GI findings; b) a prospective long term follow-up and a new upper GI endoscopy with a histological study following SBCE is mandatory in order to confirm that the so-called potentially significant lesions are not actually incidental findings, as in only 18.4% of cases gastroscopy followed SBCE findings; c) a prospective long term follow-up that takes into account possible risk factors for the presentation of non-small bowel lesions in patients undergoing SBCE and possible false positive gastroscopy procedures due to pre-SBCE mucosal aspiration, mucosal biopsies or other reasons; d) the heterogeneity of SBCE indications makes a previous conventional endoscopy unnecessary in some cases. Unlike OGIB, where conventional endoscopy should always precede capsule exploration, initial gastroscopy is not essential in other cases such as known Crohn´s disease, in which evaluation of the SB mucosa is only required to assess treatment response 33. It is likely in these cases that GDL would have been identified if an initial gastroscopy would have been indicated; e) the low rate of second-look gastroscopies (18.4%) that may confirm capsule findings and thus could be related to the benign nature of GDL. The lack of histological confirmation of findings was resolved using the diagnostic-therapeutic impact. These variables are subjective but take the global situation of the patient into account and are quite close to the specific weight of GDL detected by CE. In the current study, one gastric tumor (GIST) missed by two experienced endoscopists was detected during SBCE. In cases of OGIB with an initial negative upper endoscopy, a systematic second-look endoscopy is not recommended unless the initial endoscopy was not totally reliable. In the case presented here, the identification was difficult because the tumor was hidden between the gastric folds and was only detected by a retroversion maneuver. In another case, CE detected a duodenal adenocarcinoma distal to the papilla of Vater that was missed during the initial gastroscopy. In the remaining cases, a follicular lymphoma of the duodenum was first identified during gastroscopy and was confirmed by SBCE, while the other so-called upper GI tumors were actually submucosal lesions seen as a bulge in the lumen; and f) there is a lack of information regarding the maximum period of time between conventional endoscopic procedures and SBCE. 30 months was chosen as an arbitrary date in this study. However, it is well known that endoscopic procedures should be repeated if a prior endoscopy was unreliable. Otherwise, SBCE should be performed as soon as possible after a negative gastroscopy 8) (9.
In conclusion, upper endoscopy is the first-line procedure for the visualization of the upper GI tract. However, a careful review of these images should be performed during small intestine capsule exploration as the stomach and the duodenum are adequately visualized in the majority of cases. This allows a significant proportion of missed or underestimated lesions to be identified that may result in new diagnoses and changes in patient management. Further prospective studies are needed to confirm these results.