INTRODUCTION
Colorectal cancer (CRC) is the third most frequent cancer worldwide and ranks as the fourth leading cause of death from cancer 1. The risk of developing CRC in industrialized countries is between 5% and 6% and the mean age at diagnosis is 66 years. Over the last decade, colon cancer (CC) has been described as a very heterogeneous neoplasm from a molecular and morphological point of view, and this variability has prognostic and predictive repercussions with regard to response to treatment for metastatic (stage IV) colon cancer 2) (3.
One of the most surprising phenomena is the biological differences between cancers of the left and right side of the colon. These differences were first reported by Beart and Buffill 4) (5 in the 1980s and 1990s and were corroborated by O'Dwyer 6 in stage IV disease in 2001. This phenomenon has aroused great interest, as tumor location influences survival in both stage III and IV disease 7.
The aim of the present study was to analyze the clinical, histopathologic and oncologic differences between cancers of the left and right side of the colon in a large cohort of patients with stage I, II and III CC who underwent a homogeneous treatment.
PATIENTS AND METHODS
The study was approved by the corresponding Research Ethics Committee and the STROBE criteria were followed throughout the study. The study cohort was from a single center prospective database of CRC cases; consecutive patients who had undergone surgery for adenocarcinoma of the colon with curative intent (R0) between 2000 and 2014 were identified. All data were prospectively recorded. Patients with stage IV disease, patients who had emergency surgery due to perforation or obstruction, cases with synchronic tumors, hereditary syndromes and cases with insufficient pathologic analysis were excluded.
Right-sided tumors were defined as those located between the cecum and the splenic flexure and left-sided tumors are located between the flexure and the upper third of the rectum (> 15 cm of the anal margin). Splenic flexure tumors were considered as left-sided tumors. Up until 2008, open surgery was performed and most subsequent procedures were carried out laparoscopically, using the same oncologic criteria with proximal ligation of the vessels.
For this study, the most relevant demographic and clinical variables were collected and these included: age, sex, ASA (American Society of Anesthesiologists) grade, body mass index (BMI was calculated as weight in kilograms divided height in meters squared), Portsmouth Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (POSSUM, P-POSSUM) and main symptoms 8) (9) (10. Two authors (JLH, JB) prospectively recorded all postoperative complications, which were categorized using the Dindo-Clavien classification based on the treatment required to treat any given postoperative complication 11. Anemia was defined according to the World Health Organization (WHO) classification of anemia for the adult population (age > 15 years), hemoglobin levels less than 12 g/dl in non-pregnant women and hemoglobin levels less than 13 g/d in men 12 during the two months before surgery. Transfusion was defined as having received at least one allogeneic red blood cell transfusion within 24 hours of surgery.
Pathologic analyses were performed by a pathologist blind to the clinical progression of the patients. The norms of the American College of Pathologists 13 were followed and tumors were staged according to the TNM classification (American Joint Committee on Cancer [AJCC]) 14. The degree of differentiation and the mucinous and signet ring cell subtypes were defined according to the WHO criteria using the following three categories: well, moderately and poorly differentiated 15. Perineural invasion (PNI) was defined using the criteria of Batsakis 16 as the presence of tumor cells within any layer of the nerve sheath or clusters of tumor cells occupying more than 33% of the perineural space. Lymphovascular invasion (LVI) was confirmed using the criteria of Sato and Washington as the presence of tumor cells within the vascular space or the presence of erythrocytes or elastic lamina surrounding the tumor 17.
Operative mortality was defined as any death occurring during the 30 postoperative days. Patients were monitored using the guidelines issued by the American Society of Clinical Oncology (ASCO) 18. Adjuvant chemotherapy based on 5-fluouropirydimines was administered for six months from the fourth postoperative week to all stage III cases and those stage II cases with signs of poor prognosis (T4, fewer than 12 lymph nodes isolated and perineural and/or lymphovascular invasion) 19.
Loco-regional recurrence was defined as any histological or clinical evidence of tumor re-growth in or near the primary site after excision of the primary tumor, irrespective of the presence or absence of distant metastases. Distant recurrence was defined as clinical, radiological and/or pathological evidence of systemic disease spread outside the primary tumor basin, at sites including but not limited to the liver, lungs and paraaortic region.
The primary study end points included time to first local relapse, distant metastasis, overall survival (OS) and disease-free survival (DFS). The clinical and pathological factors outlined above were correlated with the study end points. Overall survival was defined as the time from resection to death or last follow-up. Disease-free survival was defined as the time from surgery to recurrence or death from any other cause or last follow-up.
Statistics
Statistical analysis was performed using the statistical software package SPSS statistics version 20.0 for Windows (SPSS, Chicago, IL). Univariate analysis of categorical data was performed using the Chi-squared test. Quantitative variables were expressed as the arithmetic mean/standard error and compared by the Mann-Whitney U test. The analysis of categorical data included the proportions and Chi-squared test. Survival analysis was determined by the Kaplan-Meier method and the log-rank test was used to compare statistical differences. All statistics tests were two-sided and a p-value of < 0.05 was considered to be statistically significant.
RESULTS
The demographic and clinical characteristics of the patient cohort are shown in table 1; 431 of 950 patients (45.3%) had right-sided colon cancer. The age of patients with right-sided colon cancer was significantly higher than that of patients in the left-sided group (65.1 vs 62.6; p < 0.001; OR 0.977, 95% CI 0.965-0.989). This difference was clearer in patients over the age of 75 (p < 0.001; OR 0.372 95% CI 0.256-0.540). There were more women in the right-sided group.
Anemia (n = 215, 49.8%) was the most common complaint at presentation in the right-sided group, and lower gastrointestinal bleeding (n = 208; 39.8%) and abnormal bowel movements (n = 222, 42.5%) in the left-sided group. It is noteworthy that, in our series, 23.2% and 19.3% of the patients were diagnosed via a screening program (colonoscopy and fecal occult blood test) (Table 1). No significant differences were found in the ASA, BMI, POSSUM predictive score of morbidity and mortality and the P-POSSUM operative mortality score.
With regard to perioperative parameters, surgery time was longer and more laparoscopic resections were carried out in the left-sided group (23.2% vs 36.5% respectively, 146 min vs 165 min; p < 0.001). Significantly, more blood product transfusions were performed in the right-sided group (18.8% vs 11.3%; p = 0.01, OR 0.462, 95% CI 0.313-0.682). Postoperative complications were more frequent in the resection of right-sided colon cancers (28.5% vs 21%; p = 0.004) with major complications such as Clavien-Dindo IIIb-IV in this group (Table 2). Postoperative ileus was significantly more frequent in cases of cancer in the right colon (10.2% vs 4.0%; p < 0.001). Operative mortality was 1.2% and 0.2% respectively (p = 0.07) (Table 2), a figure much lower than that predicted by the POSSUM index (3.93% and 2.6% respectively) (Table 1).
Table 1 Baseline characteristics of all patients included in the study. Right-sided colon cancer (RCC) compared to left-sided colon cancer (LCC)
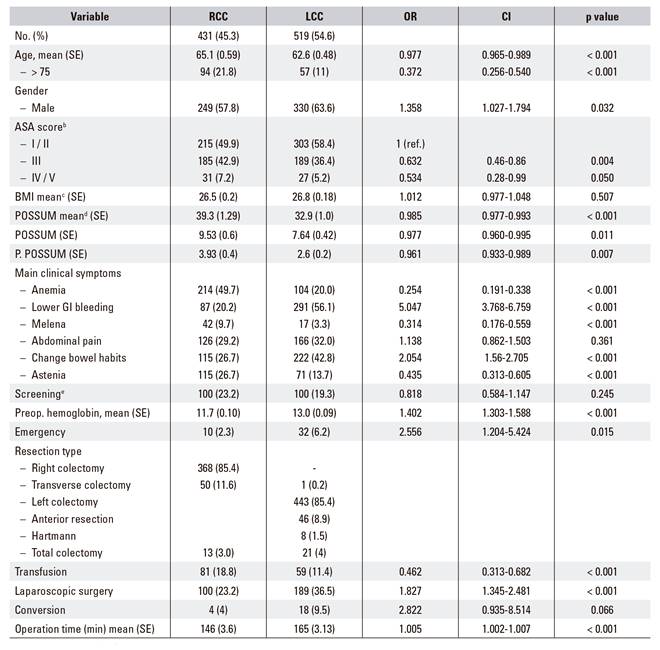
aCalculated using a χ2 test for dichotomous outcomes (percentages) and a Mann-Whitney U test for continuous outcomes. bAmerican Society of Anesthesiologist Physical Status Classification 8. cBody mass index, calculated as weight in kilograms divided by height in meters squared. dPhysiological and operative severity score for the enumeration of mortality and morbidity (POSSUM) and Portsmouth revision P-POSSUM 9) (10. eIncludes screening colonoscopies and fecal occult blood test. RCC: right-sided colon cancer; LCC: left-sided colon cancer. SE: standard error.
Tumors were significantly larger in the right colon (p < 0.001) as was the number of lymph nodes isolated (18.3 vs 15.3; p = 0.005) (Table 2). With regard to tumor staging, more early stage tumors were found in the left-sided group (p = 0.002) (Table 2). Similarly, there were significant differences in the histological phenotype between both locations, with a greater incidence of mucinous adenocarcinomas (12.1% vs 6.6%; p < 0.001) and signet ring cell carcinoma in right sided tumors. Right-sided tumors were also more undifferentiated than left-sided tumors (G3 18.1% vs 7.6%; p = 0.001), although there were no differences in the degree of perineural or lymphovascular invasion between each site.
Table 2 Histopathological analysis and outcome of all patients included in the study: right-sided colon cancer (RCC) compared to left-sided colon cancer (LCC)

aCalculated using a χ2 test for dichotomous outcomes (percentages) and a Mann-Whitney U test for continuous outcomes. bAJCC. American Joint Committee on Cancer 14. cDindo-Clavien classification of complication severity 11. RCC: right-sided colon cancer; LCC: left-sided colon cancer. SE: standard error.
The administration of adjuvant chemotherapy was similar in both groups (34.7% vs 39.3%). After a median follow-up of 103 months, the overall survival at five and ten years of right-sided colon cancer was 90.6% and 86.3% respectively and 90.8% and 85.7% respectively in left-sided tumors (Fig. 1). None of these differences between the two groups was statistically significant.
OS and DFS in stages I and II were similar when survival was analyzed according to the pathologic stage. However, in stage III tumors, the five and ten year disease free survival was higher in the left-sided group with a trend towards statistical significance (65.6% and 64.4% vs 73.9% and 70.1%, p = 0.06) (Fig. 2). When survival was analyzed in relation to chemotherapy, a similar pattern was observed, with a longer DFS in patients with left-sided tumors (Fig. 3). There were no differences with regard to local recurrence (3.2% vs 2.3%) and distal relapse (15.3% vs 13.9%) as well as the pattern of recurrence; the liver and lung were the most frequent type (Table 2).

Fig. 2 Kaplan-Meier disease-free curve for patients with right- and left-sided colon cancers (stage III).
DISCUSSION
Colorectal cancer (CRC) is the third most frequent cancer worldwide and is the fourth leading cause of death from cancer 1) (20. Since the description of the model of carcinogenesis in 1990 by Fearon and Vogelstein 2) (21, several systems to classify colon cancer have been proposed in an attempt to decipher the variability in the biological behavior of the tumor and identify molecular factors predictive of prognosis, which would allow a more personalized form of treatment. These studies have confirmed that CC is a very heterogeneous tumor 3) (22.
One of the most striking recent findings has been the differences in oncological outcomes in stage IV disease depending on whether the primary tumor is located in the right or left side of the colon 23. The first description of the differences in phenotype and clinical features of cancers of the right and left colon were provided by Beart and Bofill 4) (5 in the 1980s and 1990s, and subsequently by O'Dwyer 6 in metastatic colon cancer in 2001. The embryological, histological and physiological differences in phenotype and survival have been reported for these two locations, even though the exact biological mechanisms underlying these differences remain to be elucidated 24) (25) (26.
In this study, the morphologic and clinical differences and the oncologic outcomes of sporadic cases of colon cancer occurring at both locations was analyzed. In this series, right-sided colon cancer was significantly more frequent in women and in older patients than left-sided colon cancer, a finding which is consistent with other recent series 27) (28. The ASA stages and the predictive POSSUM and P-POSSUM indices were similar to other series and in line with most studies. We observed a tendency for these indices to over-predict the expected mortality and morbidity as compared to that actually observed 29. Postoperative morbidity was higher in the right hemicolectomies, which is in agreement with the data from several studies 30. This has been linked to older age, the need for blood products during surgery and a greater number of open surgeries at this location 30) (31.
Tumors of the right colon exhibited a higher incidence of the mucinous and signet ring cell phenotype, and were larger and more undifferentiated, which is consistent with findings from previous studies 27) (32. The incidence of PNI and LVI was similar to that reported by other studies 33 and there were no significant differences between the two groups. With regard to the analyses of survival by stage, there were no differences in OS and DFS in stages I and II. However, a poorer five and ten-year disease-free survival in tumors of the right colon was observed in stage III tumors. This finding is also in agreement with data from most other studies 34) (35) (36. A recent meta-analysis of 66 studies by Petrelli et al. 37 that included 143,846 patients (stages I, II, III) confirmed that the left-sided location of tumors was associated with better survival (HR 0.84; 95% CI, 0.79-0.89; p < 0.001). This observation was corroborated in this study, as we also found that patients with left-sided tumors responded better to chemotherapy (Fig. 3) than patients with right-sided tumors. The reasons for such disparities remain unclear even though the prognostic differences between both locations are increasingly clear (stages III and IV) 37.
The overall incidence of recurrence was similar in both groups (15.7% vs 14.6%) and, unlike other studies, there were no differences in the patterns of recurrence 38. A greater incidence of pulmonary and hepatic metastases in more distal tumors (left and sigmoid colon) has been reported in other studies. However, other studies (including the study presented here) have reported no such differences 38. The recurrence rate found in this study was similar to data reported from homogeneous single-center studies and lower than that reported by multi-center studies which include emergency cases of perforation, obstruction and insufficient lymphadenectomies (< 6 lymph nodes) 39) (40.
The molecular subtype, the presence of microsatellite instability via immunohistochemistry (MSI-H phenotype), 18q loss of heterozygosity and Coloprint, Oncotype 41 were not analyzed in this study, as cases were disease stage I, II and III. As previously mentioned, the histological patterns observed in tumors of the right colon (mucinous adenocarcinoma in 12.1% of cases and undifferentiated tumors in 18.1%) are more frequently associated with the presence of errors in the mismatch DNA repair pathway (MSI-H) 42. A groundbreaking study by Ribic et al. 43 reported that the presence of MSI-H was associated with better survival in patients with stage II and III disease who did not receive chemotherapy. A subsequent meta-analysis of 32 studies including 7,642 patients reported a better overall survival in patients with MSI-H with an HR of 0.65 (95% CI, 0.59-0.79) 44. However, other studies found no such association. It has been reported that MSI-H has a negative predictive value with regard to the benefit of adjuvant chemotherapy in stage II disease (HR 2.95; 95% CI, 1.02-8.54, p = 0.04) 45. The fact that stage II disease was most frequent in the right colon (43.1%) in our study and the frequency of MSI-H in this stage is approximately 15-20% could explain the absence of such differences in stage II disease 42. In addition, the greater incidence of complications in right-sided resections and the impact of perioperative factors on tumor biology 31) (46 could explain the differences in DFS in stage III disease. The negative impact of blood transfusions, anesthesia and postoperative complications (especially those relating to infection) on the oncologic outcome of colorectal cancer and other gastrointestinal tumors is well known. In this series, there were more complications and blood products were more frequently used in right-sided tumors as opposed to left-sided tumors, a finding that is consistent with data from several other studies (30). The basis of such an association has been proposed to be the inhibition of the cell-mediated adaptive immune response secondary to tissue damage and blood transfusions 46.
There is experimental and clinical evidence to suggest that postoperative immunosuppression stimulates the reawakening of latent circulating tumor cells or micrometastases 47. Logically, this effect would be more pronounced in tumors of more advanced stages (stage III) or more undifferentiated tumors, as in our case. The immune response to the tumor forms the basis of the systems used to classify colon cancer 48. Curiously, tumors of the right colon are characterized by the presentation of an increased infiltration of effector memory T-cells and would therefore be more susceptible to inhibition of a T-cell-mediated immune response. Other studies have linked the negative impact of perioperative complications to the release of inflammation-mediating cytokines (IL-6, IL-1, TNF-α, vascular endothelial growth factor [VEGF]) which favor the proliferation and survival of tumor cells 46) (49.
Limitations of the study include the lack of data with regard to the molecular phenotype of the tumors. It would have been interesting to determine the impact of these molecular signatures on the form of presentation and the oncologic outcome of the tumors. In spite of the different classification systems reported, the TNM system (AJCC) and morphologic factors of poor prognosis (PNI, LVI) are still used in the clinic 50.
This was a retrospective study carried out in a single center and, therefore, the conclusions may be more limited than those from controlled randomized clinical trials or studies based on extensive databases. However, this study was performed on a well-documented, wide series of cases that were treated homogeneously. As previously mentioned, the results from single center studies are superior to those from databases including multiple centers.
In conclusion, our findings confirm that differences exist in the biological behavior of cancers of the right and left colon. Such differences should be taken into account when analyzing clinical series or clinical trials, especially in stage III and IV disease. Multicenter data analyses should be explored in the future in order to test these results in CRC patients. In addition, stage IV patients were not included in our study. Further studies should be conducted to test whether the tumor location affects treatment and survival in these patients.















