INTRODUCTION
Even though endoscopic ultrasonography (EUS) has improved the early detection of small pancreatic lesions, pancreatic ductal adenocarcinoma (PDAC) is still the most lethal common cancer as it is usually diagnosed at an advanced stage 1. In fact, EUS is able to detect lesions smaller than 10 mm and to obtain samples with fine needle aspiration (EUS-FNA). During the last few years, some diagnostic methods based on the immunohistochemical analysis of pancreatic tissue have been developed. Several protease families have been studied in this setting 2 such as lysosome enzymes that are key for the degradation of proteins in the acidic environment of the lysosomes 2,3. Cathepsins play a key role in tumor induction, growth, invasion and progression by acting on the tumor cells and especially on the microenvironment 4,5,6,7,8,9,10,11,12. Several experimental and human studies have shown the expression of different cathepsins in tumor cells 13,14 as well as in serum and pancreatic juice from patients with PDAC 15,16. The role of some cathepsins, especially B, L, S and H, has been studied in the pathogenesis of cancer and their utility as prognostic markers which has been assessed in different tumors. However, the results are still controversial 14,15,17,18,19,20. In vitro studies suggest that cathepsin tissue expression might allow the use of techniques such as cathepsin-activatable near-infrared (NIRF) probes and confocal fluorescence laser microscopy (CFL) for early diagnosis 14,17,21.
To our knowledge, the expression of cathepsins in cytological samples from pancreatic EUS-FNA has not been previously reported. Therefore, a pilot study was performed in order to ascertain the expression of several cathepsin proteins in cytological samples from solid pancreatic masses obtained by EUS-FNA. Furthermore, the relationship between cathepsin expression and tumor stage was also assessed.
METHODS
Subjects
Consecutive patients that underwent EUS-FNA for a solid pancreatic lesion from March 2012 to December 2013 were included in the study. Patients that did not meet the previous requirements, with contraindications for FNA, did not sign the informed consent for the study or those with insufficient or inadequate histological material to perform the immunohistochemical studies were excluded. Informed consent was obtained at the time of the EUS-FNA procedure. All procedures were performed in accordance with the Good Clinical Practice guidelines and according to the ethical principles for medical research involving human subjects set forth by the Declaration of Helsinki. The study was approved by the local Ethics Committee.
Methods
Sample collection: EUS was performed using a linear echoendoscope (GF-UC140P, Olympus America, Inc, Center Valley, Pa). Once the lesion was localized, FNA was performed using 22-gauge needle (Boston Scientific, Natick, Mass) without the presence of on-site cytopathology. The aspirated material was extended onto several glass slides and were fixed with alcohol for a subsequent review. In addition, the clots and solid fragments were embedded in formalin for assessment by cell block preparation. Additional passes to obtain more sample was left to the discretion of the endosonographer. A final diagnosis of the lesion was determined by analyzing the surgical specimen in patients that underwent surgery, cytology, histology findings or clinical monitoring. The expression of cathepsins was also analyzed in resected surgical specimen. Lesion size and location in the pancreas were determined by the EUS findings. The stage of the malignant lesions was based on the TNM classification 22.
Material selection: After the conventional cytological staining (Hematoxilin & Eosin) determined the diagnosis of the lesion, all the cytological preparations from cell blocks were examined; besides, the most representative lesion areas were located. A tissue microarrays (TMA) was built with two punches of 1 mm of representative tissue from each patient, from cell blocks and surgical specimens of the pancreatic lesions. A TMA of normal and tumor was also prepared tissue from other organs, including the pancreas and was used to optimize (concentrations and adequate incubation time) the commercial antibodies (Ab) (case control TMA).
Immunohistochemical study:
Optimization of commercial, primary Ab: The antibodies included: Mouse cathepsin H Antibody (clone: Pol Goat IgG; dilution 1:80; supplier R&D; pretreatment: citrate buffer pH9; incubation 10 min, RT; method EnVisionFLEX). Antihuman cathepsin L Antibody (clone: Pol Goat IgG; dilution 1:80; supplier R&D; pretreatment: citrate buffer pH9; incubation 10 min, RT; method EnVisionFLEX). Human cathepsin B biotinylated Antibody (clone: Pol Goat IgG; dilution 1:40; supplier R&D; pretreatment: citrate buffer pH9; incubation 30 min, RT; method Streptavidin-HRP). Human cathepsin L biotinylated Antibody (clone: Pol Goat IgG; dilution 1:20; supplier R&D; pretreatment: citrate buffer pH9; incubation 30 min, RT; method Streptavidin-HRP). In the case of the primary Ab of cathepsins H and L, a secondary Ab (polyclonal rabbit anti-goat/HRP; DakoCytomation) was applied at a concentration of 1:80 for 10 minutes.
Immunohistochemical method and results evaluation: Immunohistochemistry was performed on 4-micron formalin-fixed and paraffin-embedded sections from the TMAs using standard techniques (DakoCytomation). The evaluation of expression levels (cytoplasm) was semi-quantitative, according to the percentage (0-100%) of neoplastic epithelial cells and the staining intensity (0,1+,2+,3+) for all the cathepsins (H,L,B and S). For the purpose of the study, staining 0-1+ was considered no expression and 2+-3+ expression/overexpression.
Variables of the study
Independent variables: demographics, definitive diagnosis of the lesion, characteristic of the lesion (size, anatomical location), outcome
Dependent variables: expression of cathepsin (B, S, L, H) in cytological and tissue samples, tumor stage at the time of EUS-FNA.
All the variables were collected in a spreadsheet designed for the purpose.
Statistical analysis
The differences in every clinical, cytological or histological and immunohistochemical variables were assessed. A relative frequency as a percentage was used for qualitative variables and mean and standard deviation or median and percentiles 25 and 75 according to the distribution type was used (parametric or non-parametric) for quantitative variables. The Chi-Square Test and Fisher's Exact Test was used to assess the correlation between the immunohistochemical data and the clinical-pathological factors, when necessary. Statistical significance was set at p < 0.05.
RESULTS
Thirty-seven FNA procedures were performed in pancreatic lesions. Adequate material for a cytological diagnosis was acquired in 35 cases (94%), this included 28 PDAC, 6 benign lesions and 1 solid pseudopapillary tumor. However, a valid sample for the immunohistochemical study was only obtained in 17 cases (48% from valid samples for cytological assessment). During the study period, six patients underwent curative surgical treatment, seven did not receive this treatment and four could not be monitored as they were from another hospital. The expression of cathepsin was also determined in the surgical specimen in three patients who underwent surgery. Table 1 shows the characteristics of the patients included in the study. All lesions with cytological material had a definitive diagnosis of adenocarcinoma. Among the 17 patients, most lesions were located in the head of the pancreas with an average size of 28 mm. As usual in this type of cancer, most tumors had already spread at diagnosis, therefore only six patients underwent a resection of the lesion (Table 1).
Table 1 Characteristics of the patients and pancreatic lesions
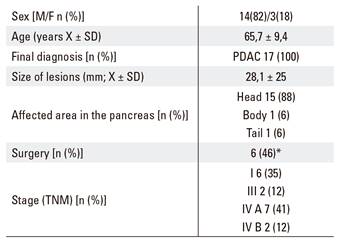
*4 patients lost during follow-up. PDAC: Pancreatic ductal adenocarcinoma.
The expression of cathepsin was generally low (Fig. 1). The expression levels of cathepsins B and S were higher (65% and 76%, respectively) than H and L (41% and 23%, respectively) in pancreatic adenocarcinomas. Nevertheless, the results in all cases were not sensitive enough to aid the diagnosis of these tumors. Of note, the expression of cathepsin was determined in both cytological and histological samples in three patients and a complete concordance of the results of cathepsins H and B were observed. However, there were discrepancies with regard to L (in one patient) and S (in two patients). Furthermore, there was no correlation between the expression of cathepsin and the extension of the neoplasia (Fig. 2). Only cathepsin B and S seemed to be expressed more frequently in more advanced stage tumors, although the differences were not statistically significant.
DISCUSSION
Generally, most PDAC tumors have spread at the time of diagnosis. In addition, the only curative treatment of this tumor is surgery, which is far from satisfactory due to the rapid dissemination of the tumor. Small lesions can be detected and cytological samples obtained by EUS-FNA. This would help to diagnose the tumor at an earlier stage and theoretically decrease the high mortality rate 23. However, EUS-FNA has some limitations, especially due to the low sensitivity. Recently, molecular biological analysis (KRAS, MUC, p53, p16, S100P, SMAD4 and microRNAs) using specimens obtained by this procedure showed an improved accuracy of the diagnosis of pancreatic carcinoma 24. Cathepsins are lysosome enzymes involved in some stages of carcinogenesis 4,5,6,7,8,9,10,11,12. Previous studies have demonstrated an intense expression of different cathepsins in pancreatic tumor tissue, ranging from 70% up to 96%. Interestingly, our group recently found a significantly higher expression of cathepsin H, L, B and S in histological material from malignant pancreatic lesions compared to premalignant or benign lesions 25. However, cathepsin expression has not been reported in cytological samples and thus, this is the strength of our study. To our knowledge, this is the first study to assess the expression of cathepsin in cytological samples obtained by EUS-FNA. Interestingly, experimental in vivo studies have shown that the use of cathepsin-sensitive probes and a confocal microscope might help to detect this tumor at an early stage 14,21. These probes could be inserted through the instrument channel of the endoscope and the suspicious areas to be sampled are selected 26. This was the purpose of the present pilot study. Although previous reports of other molecular biological approaches with pancreatic specimens obtained by EUS-FNA were encouraging 24, our results were disappointing. We are aware that the main weakness of the current study is the small number of patients included in the study, only 17 patients with PDAC. The reasons for these poor results are diverse. Firstly, as this is a pilot study, the patient sample size is small (n = 17). Attempts were made to include more patients but this was hampered due to the difficulties in obtaining valid and sufficient cytological material for the determination of cathepsin expression. Even though valid material was obtained for a conventional cytological diagnosis in 35 out of 37 FNA samples, there was insufficient material for additional immunohistochemical studies in 48% of the samples. Secondly, cathepsins are expressed both in epithelial and stromal pancreatic cells, however only epithelial cell expression was evaluated in this study. It is possible that the epithelial cells obtained by FNA were sufficient for the diagnosis, although they were not representative of the whole lesion. Furthermore, tumor heterogeneity has to be taken in consideration. In fact, we have previously reported cathepsin expression of 75% to 92% in malignant pancreatic histological material using the same technique 25. Therefore, these two factors could explain the differences in the expression of these proteases with respect to the results obtained from histological samples. In this regard, the discordance in the expression of cathepsin L and S between the cytological and histological samples from the same patients is remarkable. Despite the weaknesses of the present study, the low sensitivity restricts the role of this technique in the diagnosis of PDAC.
Generally, cathepsins play a role in oncogenesis at different levels, including tumor progression and distant dissemination 2,3,4,6,7,8,10,12,27,28. Therefore, from a theoretical point of view, the expression of these proteases is expected to be related with tumor dissemination. Accordingly, the expression of some cathepsins has been observed in positive lymph-nodes and distant metastasis 17,18. Interestingly, a correlation between the expression of cathepsin B and L with tumor extension at diagnosis and survival rate has been demonstrated 20. In contrast, we did not observe any association in our series of patients who underwent EUS-FNA. Although as previously mentioned, this may be due to the poor sample status and low sensitivity of the expression of some cathepsin proteins.
In conclusion, the expression of cathepsin in cytological material obtained by EUS-FNA of pancreatic adenocarcinoma in this pilot study is diverse and insufficient for a pre-operative diagnosis of this neoplasia. Moreover, there is no relation between the expression levels of cathepsin in the material obtained by EUS-FNA and the extension of the pancreatic tumor at diagnosis.















