Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.10 Madrid oct. 2018
https://dx.doi.org/10.17235/reed.2018.5665/2018
ORIGINAL PAPERS
Primary biliary cholangitis in Spain. Results of a Delphi study of epidemiology, diagnosis, follow-up and treatment
1Unidad de Hepatología. Hospital Clínic. IDIBAPS, CIBERehd. Universidad de Barcelona. Barcelona. Spain
2Servicio de Gastroenterología y Hepatología. Hospital Universitario Ramón y Cajal. IRYCIS, CIBERehd. Universidad de Alcalá. Madrid. Spain
3Unidad de Aparato Digestivo. Complejo Hospitalario de Especialidades Virgen de la Victoria. Málaga. Spain
4Servicio de Aparato Digestivo. Hospital Universitari i Politècnic la Fe. Universidad de Valencia. CIBEREHD. Valencia. Spain
5Servicio de Aparato Digestivo. Hospital Universitario Marqués de Valdecilla. Santander. Spain
6UGC de Aparato Digestivo. Hospital Virgen del Rocío. Sevilla. Spain
7Unidad de Hepatología. Servicio de Aparato Digestivo. Parc Taulí Sabadell. Institut d'Investigació i Innovació Parc Taulí I3PT. Universitat Autònoma de Barcelona. Sabadell, Barcelona. Spain
8Departamento Médico. Intercept Pharmaceuticals. Madrid. Spain
9Departamento Market Access. Omakase Consulting. Barcelona. Spain
INTRODUCTION
Primary biliary cholangitis (PBC) is a rare, serious and progressive autoimmune liver disease that eventually leads to liver cirrhosis, liver failure and death 1,2. PBC is characterized by serum anti-mitochondrial antibodies (AMAs) and gradual autoimmune destruction of the small bile ducts in the liver, with cholestasis and potential damage to liver tissue 1. It is also characterized by increased bilirubin levels following significant liver damage 4 and blood test abnormalities such as increased alkaline phosphatase (ALP) levels and changes in other liver damage markers including gamma-glutamyltransferase (GGT), aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) 1,2,3.
Accurately establishing PBC epidemiology is challenging. The Orphanet report of May 2015 indicated that the estimated incidence and prevalence of PBC in Europe was 30 cases/million and 210.5 cases/million people, respectively 5. The condition is 10-fold more common in females than in males 4. Every year, increasing numbers of new cases are recognized 4,5, which is likely due to improved identification of silent and asymptomatic cases as a result of an increased knowledge and improved diagnostics. Disease triggers are poorly understood and environmental factors are thought to play relevant roles in disease onset 6.
PBC usually progresses slowly in early stages. However, its course is highly variable among patients 7. Up to 30% of patients may develop a serious, progressive form of PBC with early fibrosis and liver failure 4. Some patients experience rapid progression over a period of only two years. Approximately 60-80% of patients with PBC remain asymptomatic at the time of diagnosis 8. Once symptoms develop, disease progression is rapid with a mean survival of four to eight years for asymptomatic patients 9. Without treatment, the disease progresses to cirrhosis and most patients reach the next histological stage within two years 7,10. Without treatment, the estimated mean survival time from the appearance of AMA in serum is approximately 20-22 years 11,12.
The natural history of PBC has become less severe over the past few years, mainly due to an earlier diagnosis and treatment 1. The introduction of ursodeoxycholic acid (UDCA) as a treatment strategy has considerably changed the natural history of the disease by preventing progression to cirrhosis and portal hypertension development and also lengthening the time to liver transplantation 13,14,15,16. However, patients with an inadequate response to treatment are at a higher risk for disease progression and its fallout.
In Spain, around 35-40% of patients have been shown to respond inadequately to UDCA and 5% exhibit intolerance to this drug 2. Patients with an inadequate response to UDCA are at a higher risk of disease progression and advanced disease events and also have a lower likelihood of transplant-free survival 13,17,18,19,20,21. Liver transplantation is the sole treatment available for advanced disease 22,23, with survival rates of 80% and 70% at five and ten years, respectively 24,25,26,27. The upcoming availability of newer alternatives for non-responders to UDCA, for example obeticholic acid, is therefore expected with anticipation 24,28.
Despite advances over the last few years, PBC remains a disease with many unanswered questions. In Spain, as with several other rare conditions 29, information on the size of the PBC population and the flow of PBC patients through the health system from recognition to final follow-up is limited. Specifically, epidemiological studies were carried out more than ten years ago and were also focused on selected geographic regions 30,31,32,33,34,35,36. Furthermore, there is no nation-wide data available.
The goal of this study was to address the above unanswered questions regarding PBC in Spain and to advance epidemiology estimations, identify patient flow through the public health system, elucidate presentation and diagnosis and establish the current treatment for patients with PBC in Spain.
METHODS
The study was performed using the Delphi approach 37, which was semi-structured with two rounds of consultations via telematics followed by an in-person results validation workshop. Therefore, a review of the literature was performed, which led to an initial questionnaire that was subsequently validated by four expert clinicians. The resulting questionnaire was distributed to a wider group of expert clinicians in the first round. The responses were analyzed and a second questionnaire was developed based on questions without a consensus. This was sent to the same group of expert clinicians in the second round. The results obtained from the two rounds were validated in person at a conference that was attended by the top experts in PBC management in Spain.
Review of the literature
A review of the literature was performed to identify publications on PBC epidemiology, diagnosis and treatment, as well as guidelines, recommendations, and consensus statements relevant to standard clinical practice. A search was performed of the Medline, ISI-WOK, Scopus, Cochrane Library, MEDES and ReDIris databases, as well as gray literature sources such as Google, Albi España website 38, Asociación Española para el Estudio del Hígado website 39, Orphanet 40. Publications prior to July 31st 2016 were selected.
Questionnaire development
Data from the two rounds of the questionnaires were collected online. Using the information collected from the literature review, an initial questionnaire was developed on four PBC-related aspects: epidemiology, diagnosis, follow-up and current treatment. The questionnaire used for the second round was developed to obtain consensus answers to the questions with no agreement during the first round, or to refine the results thereof.
Validation workshop
Once the two rounds of consultations were completed using online questionnaires, an in-person workshop was held to validate the results obtained that involved a reduced number of expert participants.
Expert panel
Experts in PBC were invited and recruited into a representative group of geographic regions and excellence institutions in Spain.
Statistical analysis
The statistical analysis to establish an agreement between responses was defined before the study, which was based on central tendency (median = Q2) and dispersion measures (interquartile range Q1-Q3, where 25-75% of values are found). An agreement of questionnaire responses was established when:
Information was related to standard clinical practice or individual experience (e.g., number of patients seen per year).
Responses from ≥ 90% of participants were within the interquartile range (Q1-Q3).
The difference between Q2 and Q1 and between Q3 and Q2 was < 10%.
The difference between Q2 and Q1 and between Q3 and Q2 was not deemed relevant.
Epidemiology was estimated from expert-provided data. The number of patients with PBC in the influence area of each hospital in a given autonomous community was extrapolated to the total population of that autonomous community. Extrapolated figures for each autonomous community were in turn extrapolated to the entire Spanish population using data from the Instituto Nacional de Estadística (INE) 41.
RESULTS
Review of the literature
The review of the literature yielded 51 publications. Major studies dealing with PBC epidemiology in Spain are listed in Table 1. The incidence and prevalence obtained from these publications are highly variable according to geographic area and year of publication.
Questionnaire development
The questionnaire developed for the first round included 59 items: 10 on epidemiology, 27 on patient flow and diagnostic process, 12 on diagnosis and follow-up and 10 on treatment. A second questionnaire was developed from the results obtained in the first round and items that did not reach a consensus were reformulated. The second questionnaire included 11 items: 3 on epidemiology, 3 on patient flow and diagnostic process, 4 on diagnosis and follow-up and 1 on treatment.
Validation workshop
The results obtained from the two Delphi rounds were presented and validated during a face-to-face meeting with a reduced number of specialists in the management of PBC in Spain. The results were discussed until a final consensus was reached.
Expert panel
Twenty-eight experts with various specialties (57% hepatologists, 39% gastroenterologists and 4% internists) from 13 autonomous communities were involved in both the first and second round of questionnaires (Fig. 1). The in-person workshop included seven hepatologists from centers of excellence in the management of PBC from five autonomous communities.

Fig. 1 Location of hospitals represented by participants in the Delphi study. Madrid: 1. H. Ramón y Cajal; 2. H. Puerta del Hierro; 3. H. Clínico San Carlos; 4. H. General Universitario Gregorio Marañón; 5. H. Universitario Fundación Alcorcón; 6. H. de La Princesa; 7. H. del 12 de Octubre; 8. H. Universitario La Paz; Andalusia: 9. H. Universitario Virgen de la Victoria; 10. H. Universitario Virgen del Rocío; Extremadura: 11. H. Universitario San Pedro de Alcántara; Cantabria: 12. H. Marqués de Valdecilla; Valencian Community: 13. H. La Fe; 14. H. General Universitari de València; Aragon: 15. H. Clínico Universitario Lozano Blesa; Catalonia: 16. H. Parc Taulí; 17. H. Clinic; 18. H. Universitario Vall de Hebrón; Balearic Islands: 19. H. Universitario Son Espases; Canary Islands: 20. H. Universitario Nuestra Señora La Candelaria; 21. H. Universitario de Gran Canaria Dr. Negrín; Asturias: 22. H. Universitario Central de Asturias; Castile-La Mancha: 23. H. Universitario de Albacete; Castile & Leon: 24. H. Universitario de León; Galicia: 25. H. Clínico Universitario de Santiago de Compostela.
DELPHI RESULTS
Epidemiology
Expert-provided epidemiological data are shown in Table 2. It was estimated that approximately 9,400 patients are diagnosed with PBC presently in Spain, which represents a prevalence rate of 20.2/100,000 inhabitants among the entire Spanish population. The estimated number of newly diagnosed patients annually is 1,027, which reflects an estimated incidence rate of 2.2/100,000 inhabitants. The results are variable among regions. According to 68% of responders, incidence may have changed during the last few years. This is mainly due to an increased number of early diagnoses, patients with an overlap syndrome (representing 5-15% of patients according to 89% of clinicians) and the development of more sensitive diagnostic tests. Experts agree that approximately 84% of newly diagnosed cases are females, of whom 20% are younger than 40 years at diagnosis.
Patient flow
Primary Care physicians are usually involved in initially suspecting PBC and in the referral of patients to specialists, usually a hepatologist or gastroenterologist (in approximately 90% of cases) for a confirmed diagnosis. In around 28% of cases, other specialists (internists, gynecologists, dermatologists, and rheumatologists) identify PBC due to the varied symptoms of the condition and the associated morbidity that may manifest early in the course of the disease.
The mean time to referral to a specialist is approximately nine months, with an average of four visits during this time. This time period depends upon physician training, region and other medical factors, which equates to five months for asymptomatic patients. In order to diagnose PBC, both gastroenterologists and hepatologists require an average of three patient visits over a mean period of two months (range 1.5-12 months, depending on site experience). It is estimated that 15% to 20% of individuals with PBC remain undiagnosed in Spain. Once the diagnosis is established, drug therapy is initiated as well as patient follow-up.
DIAGNOSIS
According to the experts, around 65% of patients with PBC are symptom-free at the time of diagnosis and the condition is usually recognized due to changes in biochemistry parameters during a routine checkup. Approximately 15% of individuals have symptoms, usually pruritus, whereas 5%-15% manifest more complex complaints which usually reflect an overlap syndrome.
PBC is suspected when a sustained elevation of at least six months in the alkaline phosphatase (ALP) level above 1.5 times the upper limit of the normal value (ULN) is identified, provided that other potential causes of cholestasis and liver damage are ruled out. In addition, symptomatic patients usually have pruritus and melanoderma. Common blood test alterations include the presence of antimitochondrial antibodies (AMAs) (96%) and increased levels of ALP (96%), GGT (93%) and other liver function markers. However, prolonged prothrombin time (PT) is rare during the early course of the disease. Early blood test alterations in asymptomatic patients include AMAs (100%) and increased levels of ALP (89%) and cholesterol (75%). The asymptomatic stage of PBC may last over ten years. Diagnosis confirmation requires imaging studies, usually abdominal ultrasound and occasionally a liver biopsy. These examinations may take up to two to five months.
Abdominal ultrasound is indicated when cholestasis markers are present such as increased ALP and GGT levels in order to determine the extent of liver damage and to rule out extrahepatic cholestasis and other causes of liver damage. Most consulted specialists (76%) use hepatic elastography to establish the extent of fibrosis (93%) and progression in order to estimate PBC prognosis.
Liver biopsy is used to reach a diagnosis in 10% of patients with PBC. This procedure is mainly used in AMA-negative patients with abnormal biomarker levels, which applies to 8.34% of all patients with PBC. Fourteen percent of the consulted experts do not use a liver biopsy for the diagnosis of PBC, whereas 79% will take a biopsy in selected patients.
Follow-up
The goal of patient follow-up is to assess disease activity and progression and to detect the potential development of comorbidities. This requires visiting a specialist (gastroenterologist or hepatologist) and undergoing liver panel testing in order to monitor liver function and response to treatment. These tests are performed once every six months during the symptom-free stage and 5-6 times every six months during the symptomatic stage. Follow-up duration may range from 5 to 40 years for patients who respond to treatment. Liver transplantation should be considered when signs and symptoms develop that reflect progression to an advanced stage, whilst bearing in mind the high risk for both disease-related mortality during this stage and disease recurrence following transplantation (Fig. 2).
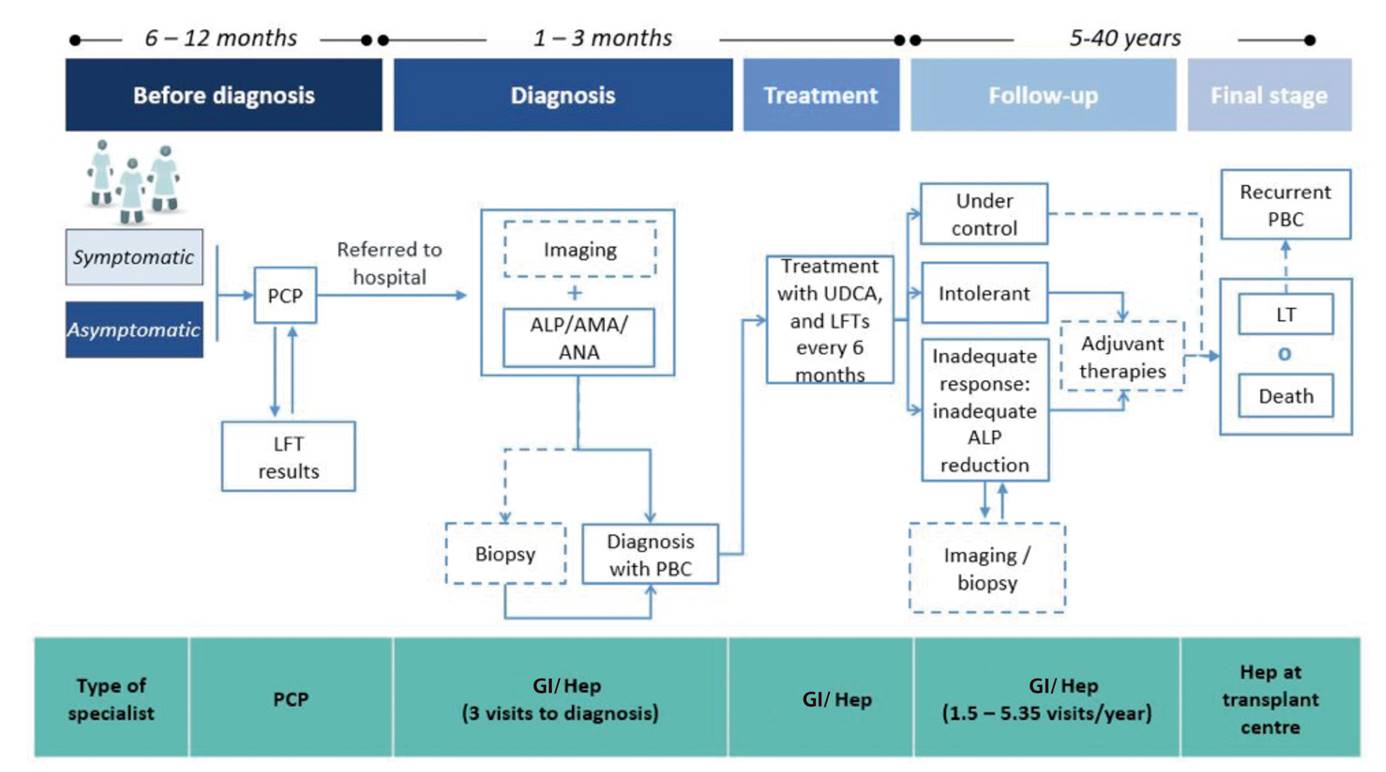
Fig. 2 Flow of PBC patients in the Spanish public health system (expert consensus). PBC: primary biliary cholangitis; ALP: alkaline phosphatase; LFT: liver function testing; AMA: anti-mitochondrial antibody; ANA: anti-nuclear antibody; PCP: Primary Care practitioner; GI: gastroenterologist; Hep: hepatologist; UDCA: ursodeoxycholic acid; LT: liver transplant. The dashed line means potential event.
Several risk factors may compromise long-term prognosis: disease stage, age at diagnosis, progression rate, prior therapies, response to treatment and the presence of an overlap syndrome. ALP, GGT, aminotransferase (alanine transaminase, aspartate transaminase) and total bilirubin levels are biochemical predictive markers that are most commonly used in medical practice. These are regularly measured during follow-up; one to two measurements per patient/yearare required during the asymptomatic stage and three to four during the symptomatic stage. Hepatic elastography and abdominal ultrasound are the most widely used imaging modalities for follow-up. The frequency of use increases with disease progression, particularly abdominal ultrasound, with a mean of 0.8 abdominal sonographies per patient/year during the asymptomatic stage and of 2.4 sonographies during the symptomatic stage.
The most common symptom experienced by patients with PBC is pruritus, which is experienced by an average of 27% of patients (range 10% to 60%) and asthenia, which affects 47% of patients (range 15% to 70%). Patients usually develop comorbidities due to PBC progression or associated conditions more than five years after diagnosis. The most common conditions in order of frequency include: Sjögren's syndrome (63% of patients with PBC), autoimmune thyroid disease (15%), osteoporosis/osteopenia (10%), Raynaud's disease (9%) and/or scleroderma (8%). Comorbidities increase the number of visits to a specialist by an average of three visits per patient per year. In addition, 2% of patients with PBC require a liver transplant in Spain. Mean transplant-free survival is 28 years after diagnosis and approximately two PBC-related deaths occur in each center each year.
Overall, 29% of specialists use validated tools to assess health-related quality of life (HRQoL). The most commonly used questionnaires include the generic instruments Short Form 36 (SF-36) (used by 14% of specialists), Fatigue Impact Scale (FIS) (used by 11% of specialists) and the specific instrument Primary Biliary Cholangitis-40 (PBC-40), which is used by 11%-14% of specialists.
Treatment of PBC
Approximately 95% of patients diagnosed with PBC receive treatment with UDCA; 3% do not receive this therapy due to intolerance (mainly gastrointestinal complaints) and 2%, due to non-adherence. The Barcelona criteria 21 are mainly used in standard practice to assess UDCA response. Specialists consider that 17% (range, 5% to 70%) of patients respond inadequately to UDCA. Considering the estimated prevalence of PBC in Spain, approximately 3,476 patients would presently be non-responders (around 39% of treated patients) and 469, intolerant to UDCA (Fig. 3). National clinical practice guidelines provide no recommendations for the treatment of patients who do not respond to or do not tolerate UDCA. Hence, a second-line treatment remains to be established. However, in the absence of an approved therapy for this indication at the time of the study -obeticholic acid received regulatory approval by the European Medicines Agency on December 12, 2016 for the treatment of primary biliary cholangitis (also known as primary biliary cirrhosis) in combination with ursodeoxicholic acid (UDCA) in adults with inadequate response to UDCA, or in monotherapy in adults intolerant to UDCA (28)-, specialists treat these patients with fibrates (administered to 57% of non-responders and 32% of intolerants to UDCA) and glucocorticoids or corticosteroids such as budesonide or prednisolone (administered to 11% of non-responders and 18% of intolerants to UDCA). None of these treatments are used on-label.

Fig. 3 Estimated number of potential PBC patients eligible in Spain to receive second-line or alternative-to-UDCA therapies (expert consensus). PBC: primary biliary cholangitis; UDCA: ursodeoxycholic acid. Population (2016): 46.4 million.
The most commonly used drugs against pruritus include antihistamines, anion exchange resins (e.g., cholestyramine), antidepressants, rifampicin and opioid antagonists. Furthermore, drugs usually administered to treat comorbidities include: diuretics (furosemide and spironolactone) to reduce ascites, beta-blockers in the case of esophageal varices to prevent bleeding, somatostatin or vasopressin in the case of gastrointestinal bleeding from portal hypertension and lactulose and rifaximin to manage hepatic encephalopathy.
Experts consider that standard guidelines are much needed in Spain as criteria and clinical practice are highly variable, which is in turn conditioned by the disparity in experience, personnel and technical resources between hospitals.
DISCUSSION
This study provides a summary on the expert views about PBC epidemiology, patient flow, diagnosis, follow-up and treatment in Spain. Participants in the study provided highly variable incidence and prevalence estimations, with a prevalence ranging from 11.79 to 37.99 cases/100,000 inhabitants and an incidence ranging from 0.51 to 3.86 cases/100,000 inhabitants; however, these results are similar to those found in the literature of studies performed in other countries and when considering the whole of Europe 5,42,43. Dispersion may be accounted for either by a wide variability in the number of patients diagnosed with PBC in each region or by epidemiological factors such as age, race, immigration status (data not analyzed), education and/or lack of a PBC registry in hospitals.
Experts agreed on a growing tendency to diagnose PBC early, which helps improve patient prognosis as treatment starts earlier. Here, the role of Primary Care physicians is key, as they are the practitioners who most often suspect the condition and refer patients to a specialist for diagnostic confirmation and treatment 44,45,46. In order to estimate disease stage and progression, liver fibrosis is routinely assessed using noninvasive techniques such as liver elastography in the hospital setting. However, limited access and availability of these procedures, as well as lack of clinical guidelines, represent barriers to their widespread use 47.
At the time of the study, UDCA was the only available alternative approved for the treatment of PBC in Spain. Hence, prognosis relies on patient response to this drug 32. While treatment with UDCA is seemingly well established, it is important to study the variability of its use in clinical practice, as this variability has been shown to influence the natural history of the disease 33. In intolerant patients or patients that respond inadequately to UDCA, other off-label therapies are used with highly heterogeneous criteria that depend on the views held by each health professional and center. Second-line therapies commonly used by experts in Spain are consistent with the off-label options included in the latest EASL guidelines on PBC diagnosis and treatment, although they are not recommended as no evidence is available to support their effectiveness 48. Thus, there is a need for approved second-line options for the management of PBC, with known benefits in terms of clinical and biochemical variables, transplant-free survival, reduced mortality from liver disease and improved health-related quality of life 30.
The limitations of the present study derive from the method used to obtain information as data reports are virtually nonexistent. The Delphi approach allows information to be collected based on the opinions of experts who treat patients with PBC, hence it requires corroboration by specifically designed epidemiologic studies. The data included in the ColHai registry (Registro Nacional de Enfermedades Colestásicas y Autoinmunes Hepáticas, started in 2016) 31 may provide more evidence to the presently available information, which is very low. One limitation that is potentially associated with the Delphi approach is the low variability among answer options, which would prevent an appropriate consideration of alternate viewpoints among participants. In order to avoid this issue, all the items included in the questionnaires used for the present study, even those with ranges or lists, had an open field for experts to insert their own data.
In conclusion, interventions directed to educate health care teams on the characteristics and needs of patients with PBC may increase diagnosis rates and shorten time to diagnosis. The new ColHai registry will allow data collection and PBC monitoring in Spain. However, Spanish clinical practice guidelines on the management of PBC that define diagnostic, therapeutic and patient follow-up criteria would also be necessary to inform, facilitate and standardize clinical decision making. In addition to gaining an insight into the health outcomes obtained with the aforementioned interventions.
ACKNOWLEDGEMENTS
We are grateful to doctors Agustín Albillos, Raúl Andrade, Carmen Álvarez, Ana del Carmen Arencibia, Marina Berenguer, Lucía Bonet, Javier Crespo, Francisca Cuenca, Moisés Diago, Conrado Fernández, Miguel Fernández, Luisa García, Miren García, Elena Gómez, Carmen González, Concepción González, Francisco Jorquera, Esther Molina, José Luis Montero, Antonio Olveira, Albert Parés, Ildefonso Quiñones, Manuel Romero, María Magdalena Salcedo, Miguel Ángel Simón, José Manuel Sousa, María Trapero, Víctor Vargas, Mercedes Vergara, and María del Mar Vicente for their participation in the present study.
Thanks also to John Shepherd for his contribution to the writing of the manuscript.
REFERENCES
1. Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev 2014;13(4-5):441-4. DOI: 10.1016/j.autrev.2014.01.041 [ Links ]
2. Parés A. Treatment of primary biliary cirrhosis: is there more to offer than ursodeoxycholic acid? Clin Liver Dis 2014;3(2):29-33. [ Links ]
3. Nguyen DL, Juran BD, Lazaridis KN. Primary biliary cirrhosis. Best Pract Res Clin Gastroenterol 2010;24(5):647-54. DOI: 10.1016/j.bpg.2010.07.006 [ Links ]
4. Corpechot C, Chrétien Y, Chazouillères O, et al. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J Hepatol 2010;53(1):162-9. DOI: 10.1016/j.jhep.2010.02.019 [ Links ]
5. Orphanet Report Series. Prevalencia de las enfermedades raras: datos bibliográficos enfermedades listadas por orden de prevalencia o incidencia decreciente o por número de casos publicados. Citado 12 diciembre 2016. Disponible en: http://www.orpha.net/orphacom/cahiers/docs/ES/Prevalencia_de_las_enfermedades_raras_por_prevalencia_decreciente_o_casos.pdf [ Links ]
6. Juran BD, Lazaridis KN. Environmental factors in primary biliary cirrhosis. Semin Liver Dis 2014;34(3):265-72. [ Links ]
7. Kumagi T, Heathcote EJ. Primary biliary cirrhosis. Orphanet J Rare Dis 2008;3(1):1. DOI: 10.1186/1750-1172-3-1 [ Links ]
8. Kurtovic J, Riordan SM, Williams R. The natural history of asymptomatic primary biliary cirrhosis. QJM 2005;98(5):331-6. A DOI: 10.1093/qjmed/hci058 [ Links ]
9. Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology 2009;50(1):291-308. DOI: 10.1002/hep.22906 [ Links ]
10. Al-Harthy N, Kumagi T. Natural history and management of primary biliary cirrhosis. Hepat Med 2012;4:61-71. [ Links ]
11. Leuschner U, Manns MP, Eisebitt R. Ursodeoxycholic acid in the therapy for primary biliary cirrhosis: effects on progression and prognosis. Z Gastroenterol 2005;43(9):1051-9. DOI: 10.1055/s-2005-858281 [ Links ]
12. Metcalf J V, Mitchison HC, Palmer JM, et al. Natural history of early primary biliary cirrhosis. Lancet 1996;348(9039):1399-402. DOI: 10.1016/S0140-6736(96)04410-8 [ Links ]
13. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008;48(3):871-7. DOI: 10.1002/hep.22428 [ Links ]
14. Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology 2014;147(6):1338-49.e5. [ Links ]
15. Poupon RE, Lindor KD, Cauch-Dudek K, et al. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997;113(3):884-90. DOI: 10.1016/S0016-5085(97)70183-5 [ Links ]
16. Corpechot C, Carrat F, Bahr A, et al. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology 2005;128(2):297-303. DOI: 10.1053/j.gastro.2004.11.009 [ Links ]
17. Angulo P, Lindor KD, Therneau TM, et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver 1999;19(2):115-21. DOI: 10.1111/j.1478-3231.1999.tb00020.x [ Links ]
18. Carbone M, Mells GF, Pells G, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013;144(3):560-9.e7. DOI: 10.1053/j.gastro.2012.12.005 [ Links ]
19. Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol 2010;105(10):2186-94. DOI: 10.1038/ajg.2010.216 [ Links ]
20. Momah N, Silveira MG, Jorgensen R, et al. Optimizing biochemical markers as endpoints for clinical trials in primary biliary cirrhosis. Liver Int 2012;32(5):790-5. DOI: 10.1111/j.1478-3231.2011.02678.x [ Links ]
21. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006;130(3):715-20. DOI: 10.1053/j.gastro.2005.12.029 [ Links ]
22. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016;37(1):67-119. [ Links ]
23. Kashyap R, Safadjou S, Chen R, et al. Living donor and deceased donor liver transplantation for autoimmune and cholestatic liver diseases - An analysis of the UNOS database. J Gastrointest Surg 2010;14(9):1362-9. DOI: 10.1007/s11605-010-1256-1 [ Links ]
24. Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375(7):631-43. [ Links ]
25. Akamatsu N, Sugawara Y. Primary biliary cirrhosis and liver transplantation. Intractable Rare Dis Res 2012;1(2):66-80. [ Links ]
26. Huang YQ. Recent advances in the diagnosis and treatment of primary biliary cholangitis. World J Hepatol 2016;8(33):1419-41. DOI: 10.4254/wjh.v8.i33.1419 [ Links ]
27. Chalifoux SL, Konyn PG, Choi G, et al. Extrahepatic manifestations of primary biliary cholangitis. Gut Liver2017;11(6):771-80. DOI: 10.5009/gnl16365 [ Links ]
28. EMA (European Medicines Agency). Summary of Product Characteristics. Ocaliva; 2016. Citado 12 nov 2017. Disponible en: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004093/WC500218418.pdf [ Links ]
29. Ramalle-Gómara E, Ruiz E, Quiñones C, et al. General knowledge and opinion of future health care and non-health care professionals on rare diseases. J Eval Clin Pract 2015;21(2):198-201. DOI: 10.1111/jep.12281 [ Links ]
30. Pla X, Vergara M, Gil M, et al. Incidence, prevalence and clinical course of primary biliary cirrhosis in a Spanish community. Eur J Gastroenterol Hepatol 2007;19(10):859-64. DOI: 10.1097/MEG.0b013e328277594a [ Links ]
31. Registro Nacional de Enfermedades Colestásicas y Autoinmunes Hepáticas (ColHai) - AEEH. Citado 28 nov 2017. Disponible en: http://aeeh.es/2016/11/registro-nacional-de-enfermedades-colestasicas-y-autoinmunes-hepaticas-colhai/ [ Links ]
32. Caballero Plasencia AM, López Callejas C, Valenzuela Barranco M, et al. Epidemiology of primary biliary cirrhosis in the south area of Granada. Med Clin (Barc) 1991;96(13):481-5. [ Links ]
33. Moreno Sánchez D, Cassinello Ogea C, González Blanco P, et al. Epidemiology of primary biliary cirrhosis in southern Madrid. Med Clin (Barc) 1990;94(15):564-9. [ Links ]
34. Borda F, Huarte MP, Zozaya JM, et al. Primary biliary cirrhosis in Navarra. An Med Interna 1989;6(2):63-6. [ Links ]
35. Parés A, Bruguera M, Rodés J, et al. Epidemiology of primary biliary cirrhosis in Cataluña. Med Clin (Barc) 1984;82(6):237-41. [ Links ]
36. Rodrigo Sáez L, Valerdiz Casasola CS, Rodríguez García M, et al. Epidemiology of primary biliary cirrhosis in Asturias. Med Clin (Barc) 1987;88(12): 486-9. [ Links ]
37. De Villiers MR, De Villiers PJT, Kent AP. The Delphi technique in health sciences education research. Med Teach 2005;27(7):639-43. DOI: 10.1080/13611260500069947 [ Links ]
38. Albi España. Asociación para la Lucha contra las Enfermedades Biliares Inflamatorias. Citado 28 nov 2017. Disponible en: http://www.albi-espana.org/ [ Links ]
39. AEEH. Asociación Española para el Estudio del Hígado. Citado 28 nov 2017. Disponible en: http://aeeh.es/ [ Links ]
40. Orphanet. Orphan Drugs in Europe. Citado 3 feb 2017. Disponible en: http://www.orpha.net/consor4.01/www/cgi-bin/Education_AboutOrphanDrugs.php?lng=EN&stapage=ST_EDUCATION_EDUCATION_ABOUTORPHANDRUGS_EUR [ Links ]
41. INE. Instituto Nacional de Estadística. Citado 13 jun 2017. Disponible en: http://www.ine.es/ [ Links ]
42. Boonstra K, Kunst AE, Stadhouders PH, et al. Rising incidence and prevalence of primary biliary cirrhosis: a large population-based study. Liver Int 2014;34(6):e31-8. DOI: 10.1111/liv.12434 [ Links ]
43. Podda M, Selmi C, Lleo A, et al. The limitations and hidden gems of the epidemiology of primary biliary cirrhosis. J Autoimmun 2013;46:81-7. DOI: 10.1016/j.jaut.2013.06.015 [ Links ]
44. Rautiainen H, Salomaa V, Niemelä S, et al. Prevalence and incidence of primary biliary cirrhosis are increasing in Finland. Scand J Gastroenterol 2007;42(11):1347-53. DOI: 10.1080/00365520701396034 [ Links ]
45. Wang L, Gershwin ME, Wang FS. Primary biliary cholangitis in China. Curr Opin Gastroenterol 2016;32(3):1. A DOI: 10.1097/MOG.0000000000000257 [ Links ]
46. Myers RP, Shaheen AAM, Fong A, et al. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population-based study. Hepatology 2009;50(6):1884-92. DOI: 10.1002/hep.23210 [ Links ]
47. Jones DEJ, Sutcliffe K, Pairman J, et al. An integrated care pathway improves quality of life in primary biliary cirrhosis. QJM 2008;101(7):535-43. DOI: 10.1093/qjmed/hcn043 [ Links ]
48. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J Hepatol 2009;51(2):237-67. DOI: 10.1016/j.jhep.2009.04.009 [ Links ]
49. Comisión Interministerial de Precios de los Medicamentos. Nota informativa de la reunión de la comisión interministerial de precios de los medicamentos. Sesión 177 de 6 de noviembre de 2017. Citado 20 mar 2018. Disponible en: https://www.msssi.gob.es/profesionales/farmacia/pdf/ACUERDOS_DE_LA_CIPM_177_web.pdf [ Links ]
50. AEMPS. Ficha técnica Ocaliva 5 mg comprimidos recubiertos con película. Citado 15 mar 2018. Disponible en: https://cima.aemps.es/cima/dochtml/ft/1161139001/FT_1161139001.html [ Links ]
Received: May 05, 2018; Accepted: May 08, 2018











 texto en
texto en 




