Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 no.11 Madrid nov. 2018
https://dx.doi.org/10.17235/reed.2018.5289/2017
ORIGINAL PAPERS
A clinical trial comparing propofol versus propofol plus midazolam in diagnostic endoscopy of patients with a low anesthetic risk
1Servicio de Aparato Digestivo. Hospital Virgen de la Concha. Zamora. Spain
2Servicio de de Investigación. Hospital Virgen de la Concha. Zamora. Spain
INTRODUCTION
The use of sedatives in gastrointestinal endoscopies is known to reduce patient discomfort and anxiety as well as increasing tolerance and acceptance. This in turn increases the satisfaction of the endoscopist due to the reduction in patient movement, which allows for a better visualization of the mucosae and also decreases therapeutic risk 1. In the absence of a specific sedation protocol, numerous studies have been conducted with currently available sedatives and relevant guidelines have been published 1,2.
Benzodiazepines, either alone or combined with opioids, have been used for decades and constitute the traditional regimen for sedation in the clinical practice 3,4. More recently, propofol has gained popularity due to its pharmacokinetic characteristics. Despite the extensive use of propofol, caution should be taken when administrated by non-anesthetists as there is no antagonist and there is also a potential to induce general anesthesia. Although, its safety in the hands of trained endoscopists has been demonstrated in numerous studies 5,6,7,8,9,10,11,12.
In our experience, performing diagnostic gastroscopies with low doses of midazolam in combination with propofol can provide advantages, including both the reduction of the required dose of propofol without increasing adverse effects as well as maintaining high levels of patient satisfaction. From this perspective, this study was performed to compare two strategies for intravenous (IV) sedation in diagnostic upper gastrointestinal endoscopy (UGE).
PATIENTS AND METHODS
A prospective, randomized and double-blind study was performed in which the use of propofol alone was compared with the use of midazolam plus propofol in a diagnostic upper GI endoscopy (UGE) diagnoses. The main objectives were to evaluate safety and efficiency, while the secondary objectives included the evaluation of the quality of the endoscopy and patient satisfaction as follows:
• Safety (frequency of the following complications: hypoxemia, bradycardia and hypotension). Systolic blood pressure below 80 or a 30% decrease from the baseline value was considered as hypotension. A decrease greater than or equal to 20% of the normal heart rate was considered as bradycardia. Hypoxia was defined as oxygen saturation below 90%.
• Efficiency (the time lapse between the initial administration of any type of sedation IV and patient discharge from the hospital). The following parameters were measured and recorded during the procedure in order to evaluate the impact of sedation on efficiency in our Endoscopy Unit:
Induction time, defined as the time elapsed between initiating sedation and beginning the exploration.
Sedation time, the time between the administrations of the first sedation dose to the final dose.
Duration of the procedure, defined as the time between the passage of the endoscope through the throat until its complete removal.
Total time of the UGE from the initiation of sedation to the complete removal of the endoscope.
Recovery time, the time between the end of the endoscopy procedure and patient discharge.
Overall duration of the procedure, defined as the time elapsed from the administration of the first sedation dose until recovery and patient discharge.
• Quality of the endoscopy (the evaluation and score of the exploration by two endoscopists according to the appropriate visualization). Two independent physicians evaluated the exploration, the endoscopist who carried out the endoscopy and a second gastroenterologist who was present during the procedure but not involved in the endoscopic process. A non-validated scale was used as a reference as described by Meining et al. (13) in 2007. The following steps (or parameters) were identified during the performance of UGE: P1: passage of the endoscope through the throat; P2: visualization of the esophagus; P3: assessment of the proximal cardiac folds; P4: passage through the stomach towards the pylorus along the greater curvature; P5: passage through the pyloric sphincter; P6: a complete evaluation of the duodenal bulb; P7: introduction of the scope and visualization of the descending duodenum; P8: complete evaluation of duodenal folds; P9: a complete visualization of the antrum; P10: visualization of the angular incisures; P11: execution of the retroflexion maneuver; P12: retroflexion visualization of the fundus and cardia; P13: visualization of the body and lesser curvature and P14: removal through the esophagus.
• Each step of the endoscopy was evaluated subjectively by the endoscopists on a quality scale ranging from 1 to 6 (6 excellent; 5 good; 4 moderate; 3 sufficient; 2 poor and 1 unacceptable) with the purpose of discovering if the quality of the endoscopy was significantly influenced by the sedative used. The overall scores for the complete examination of the three separate organs (esophagus, stomach and duodenum) were also included in the evaluation as follows: P15: overall general assessment of the upper gastrointestinal tract; P16: general assessment of the esophagus; P17: general assessment of the stomach and P18: general assessment of the duodenum.
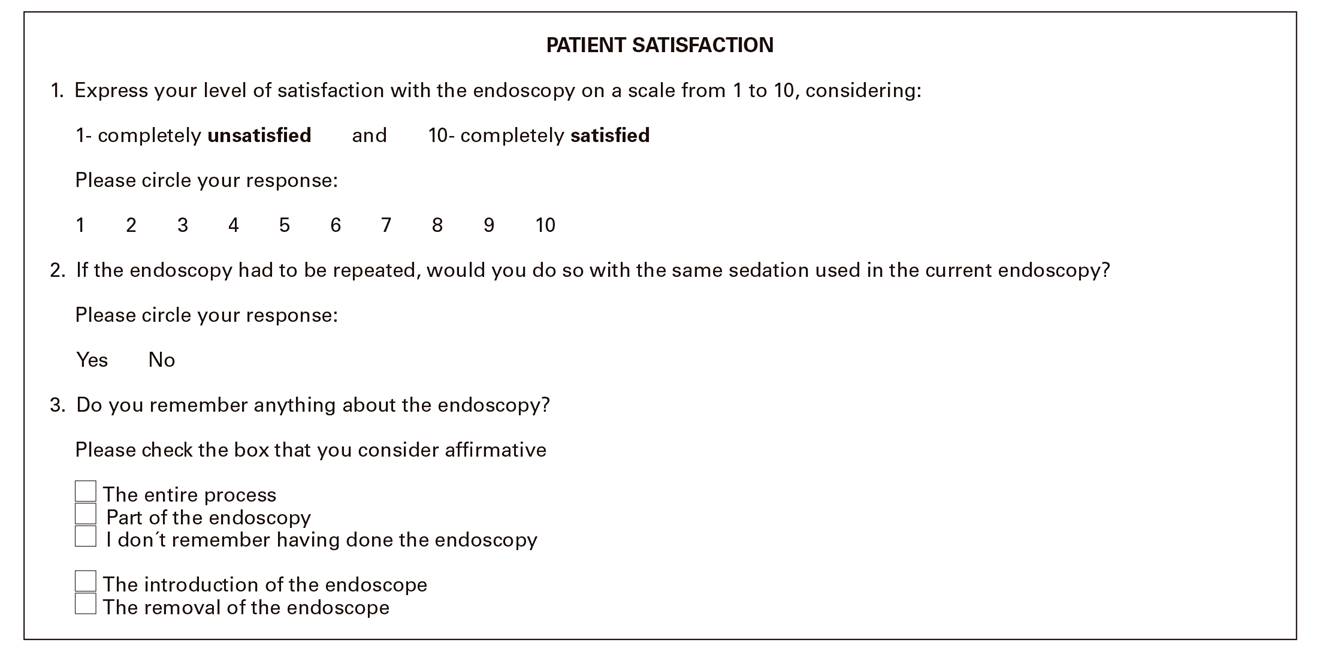
• Patient satisfaction (the opinion of the patient was expressed in a questionnaire that was completed before discharge). In order to measure patient comfort after the examination, participants were given a survey to be completed before discharge with regard to the quality of sedation and the general experience during the procedure. A visual analogical scale was used that ranged from zero (very unsatisfactory) to 10 (completely satisfactory). This questionnaire also evaluated the level of amnesia and when appropriate, the patients' willingness to undergo the same procedure in the future (Fig. 1).
Selection of patients
Eighty-three patients who were scheduled to undergo a diagnostic UGE were included in the study over a period of two months. The age range of the participants was from 18 to 80 years of age and the anesthetic risk classification was ASA I-II. The criteria for exclusion included pregnancy, alcohol and drug abuse, a relevant diagnosed respiratory illness, sleep apnea syndrome, heart failure, patients who had been administered some form of sedative up to 24 hours before the procedure and those who refused to sign the written informed consent.
Sedation protocols
The patients were randomized to receive sedation with either propofol (group A) or midazolam plus propofol (group B) on the basis of a computer-generated list. The randomization was performed using the Epidat 3.0 program. Neither topical oral anesthetics nor reversal agents were administered to any patient. In order to ensure a blinded randomization sequence, the group assignment was transferred to numbered envelops which remained sealed until the moment of sedation. Once the written informed consent had been signed (to undergo the endoscopic procedure, sedation and study participation), a sealed envelope was assigned to each patient which was opened by the nurse in charge of administering the sedative(s). This nurse, who was present throughout the procedure, prepared and carried out the sedation process. Group A received an IV placebo bolus (saline solution) while group B received an IV bolus of 3 mg of midazolam. Following the initial bolus (blinded to the patient and endoscopists), both groups were administered a bolus of 20 mg of propofol. Afterwards, boluses of 20 mg of propofol were administered upon the endoscopists´ request throughout the procedure, in order to achieve and maintain the appropriate sedation. The desired sedation was established at level 2-3 in accordance with the observers´ assessment of alertness/sedation [OASS] 13.
Endoscopic procedure, patient monitoring and data collection
The endoscopic procedures were performed by nine experienced endoscopy specialists of the endoscopic unit. During each of the endoscopies, one specialist performed the endoscopy while the other observed and evaluated the procedure. All of the endoscopies were performed using the Olympus GIF 140(r) or GIF 160(r) endoscope. Patients received oxygen supplementation via a nasal catheter from five minutes before the initiation of sedation until the removal of the endoscope. Monitoring included the non-invasive measure of blood pressure (basal, after the removal of the endoscope and before leaving the recovery room), heart rate and oxygen saturation using a pulse oximeter continuously. A gastroenterologist who did not perform the endoscopy but evaluated the procedure, was also responsible for collecting data relevant to the administered sedation, the aforementioned specified time intervals and the physiological parameters of the patient.
Patient recovery
Immediately following the procedure, patients were transferred to a recovery room. The recovery time following the procedure was measured using Aldrete's scoring system 15 at 10 minute intervals after the procedure. Patients who achieved a score equal to or greater than 9 were considered suitable for discharge.
Ethical considerations
The study protocol was approved by the Ethics Committee of our hospital (code 201). The trial was performed under conditions that conform with the fundamental rights of the individual and the ethical procedures related with biomedical research with human subjects. The study complied with the contents of the Declaration of Helsinki and its subsequent revisions. All patients signed three separate detailed informed consent forms, one for the gastroscopy, another for the administration of the sedative and a third for participation in the study.
Statistical analysis
Forty subjects per group were considered as necessary to detect a difference of 5 minutes in the global time required for the procedure (standard deviation of 8 minutes) with alpha and beta errors of 0.05 and 0.20. In addition, a sample of this size would allow the detection of differences in induction times of 1 minute (standard deviation of 1.5 minutes) 16,17. Data was analyzed using SPSS 11.5.1 and Epidat 3.0. Descriptive statistics of all the variables were determined. Quality variables between the two groups were compared using Pearson's chi-squared test, Student's t-test and ANOVA tests for qualitative and quantitative variables. The variables with measurements that were repeated over time were analyzed with the ANOVA test. Quantitative variables were evaluated using the Pearson correlative coefficient. If the distribution of the variable was not normal, non-parametric models were used. A p-value less than 0.05 was considered as statistically significant.
RESULTS
Patients and medication used
Eighty-three patients were included in the study and randomized. Of these, 42 received the placebo plus propofol (group A) and 41 received midazolam plus propofol (group B). There were no significant differences between the two groups in terms of age, weight, sex and ASA (Table 1). The most common symptoms that led to an UGE exploration were epigastric pain and dyspepsia and there were no significant differences between the two groups. The propofol dose was significantly lower (p < 0.01) in group B patients (midazolam plus propofol) in comparison to group A patients (placebo plus propofol). The average dose of propofol was 115.90 mg (SD 40.57) in group A and 83 mg (SD 40.34) in group B (Table 1).
Safety profile
Interruption of the endoscopic procedure was necessary in only four cases and all were group B cases; two were due to accidental extravasation of the intravenous catheter and intolerance and agitation in two patients. The endoscope was removed in all cases and the group to which the patient belonged was revealed (A or B) and they received a flumazenil injection. All of these patients were included in the intention to treat analysis. However, some of the data could not be completed due to the premature removal of the endoscope.
Complications were not statistically different between the groups (Table 1). The complications of both groups were handled satisfactorily and conservatively. Three patients (7.3%) in group A and four patients (9.8%) in group B had temporary low oxygen saturation levels that were less than or equal to 90%. The lowest registered oxygen saturation level was 79%. There was no difference between the groups in terms of the frequency of temporary low oxygen saturation (p = 0.15), which was managed using the jaw thrust maneuver and increasing the flow of administered oxygen. The incidence of bradycardia was higher in group A (7 patients; 17.1%) than in group B (3 patients (7.5%) and the difference was not statistically significant (p = 0.09). Hypotension affected 3 and 4 patients (7.3% and 10%) in groups A and B, respectively, which was not statistically significant (p = 0.18). All of the episodes of hypotension and bradycardia were resolved with no therapeutic intervention.
Analysis of time in relation to the procedure
The average induction time (time from commencing sedation to the initiation of the exploration) in the placebo plus propofol group (group A) was 4.07 minutes (SD 1.65) and 3.00 minutes (SD 1.30) in the midazolam plus propofol group (group B), which was statistically significant (p = 0.001). However, the times of the duration of the procedure, recovery and overall duration of the UGE were not significantly different (Fig. 2).

Fig. 2 Average time expressed in minutes of Induction time: defined as the time elapsed between initiating sedation and beginning the exploration. Sedation time: the time between the administrations of the first sedation dose to the final dose. Duration of the procedure: defined as the time between the passage of the endoscope through the throat until its complete removal. Total time of the UGE from the initiation of sedation to the complete removal of the endoscope. Recovery time: the time between the end of the endoscopy procedure and patient discharge. Overall duration of the procedure: defined as the time elapsed from the administration of the first sedation dose until recovery and patient discharge (m: minutes; ns: not significant).
Post-procedure evaluation: quality of the exploration and patient satisfaction
The quality of the exploration according to the score of the endoscopic evaluation evaluated by physicians was similar and there were no significant differences between the two groups, regardless of the sedative administered (Table 2). The acceptance rate of the patients was similar in both groups. The average satisfaction score was 9.85 and 9.92 (SD 0.52 and 0.35) out of 10 in groups A and B, respectively. In group A, 3 patients (7.3%) had some memory of the endoscopy; one remembered the entry of the endoscope and two remembered the removal. None of the patients in group B remembered the endoscopy. Although it was not an objective of the study a priori, it is worth noting that both groups had similar amnesic properties. Forty patients (97.6%) in group A and 39 patients (100%) in group B expressed their willingness to repeat the procedure in the future if it were necessary, indicating their satisfaction with the administered sedation regimen.
Table 2 The median scores awarded by each physician in each step of the endoscopy

P1: passage of the endoscope through the throat; P2: visualization of the esophagus; P3: assessment of the proximal cardiac folds; P4: passage through the stomach towards the pylorus along the greater curvature; P5: passage through the pyloric sphincter; P6: a complete evaluation of the duodenal bulb; P7: introduction of the scope and visualization of the descending duodenum; P8: complete evaluation of duodenal folds; P9: a complete visualization of the antrum; P10: visualization of the angular incisures; P11: execution of the retroflexion maneuver; P12: retroflexion visualization of the fundus and cardia; P13: visualization of the body and lesser curvature and P14: removal through the esophagus. P15: overall general assessment of the upper gastrointestinal tract; P16: general assessment of the esophagus; P17: general assessment of the stomach and P18: general assessment of the duodenum. Score range: 1-6 (1: unacceptable-6: excellent). ns: not significant.
DISCUSSION
In recent years, many studies and meta-analyses have compared traditional sedation with sedation based on the administration of propofol 18,19,20. Some disadvantages of propofol such as over-sedation, negative cardio-respiratory effects or pain in the injected area, especially if the vein used is small 21,22,23,24, have led to the study of propofol in association with small doses of opioids or benzodiazepines. These evoke a synergetic action, without increasing the side effects and reduce the total required dose of propofol 25,26. This protocol, which aims to achieve a moderate-profound sedation level, has been described in the recent medical literature as "balanced propofol sedation" (BPS) 27,28,29 and has been compared to propofol administration as the only sedative 29,30,31,32,33.
The combination of midazolam and propofol in our study diminished the required dose of propofol significantly. Group A were administered 115 mg, compared to an average of 83 mg in group B. However, the average dose of propofol in group A (115 mg) was lower than that reflected by other studies in which propofol was the only sedative agent 13,29,34,35. Although, it must be noted that their patients achieved a deep sedation level. With regard to midazolam, there was a tendency to use lower doses in most other studies than that used in this study in combination with propofol 12,29,30. The dose of midazolam in the present study was established based on the current overall experience of the professionals within our unit. The same is true with regard to the chosen dose of propofol.
When both sedation regimens were compared, we found that the adverse effects were scarce and hardly relevant and comparable between the groups. Depressed respiration and adverse cardiovascular effects such as hypotension or bradycardia were principally associated with propofol. Bradycardia constituted the most frequent adverse effect in our series, particularly in the propofol group and occurred in 7 (17.1%) and 3 (7.5%) patients in groups A and B, respectively. This data proves the safety of both regimens previously mentioned in other reports which compared the administration of propofol alone with BPS 12,29,30,31,32,33.
Our data shows that the BPS protocol that combines low doses of midazolam with propofol (group B), achieved a quicker sedation than propofol administered as monotherapy (group A). Although the induction time difference was statistically significant, it was an isolated element as there were no other statistically significant differences in the duration of the procedure, recovery time or the overall duration of the UGE. The induction time with midazolam plus propofol (BPS) in group B was three minutes and was similar 27,28 or slightly higher 29,30,33 than that reported by other studies which also used BPS. Cohen et al. 36 found that the addition of narcotic and benzodiazepine to propofol did not appreciably alter the induction time compared with other studies which used only propofol. In group A (placebo plus propofol), the sedation induction time (4.07 minutes) was very similar to that described in other studies 30,33,18. However, in general, data collected from other studies that used propofol as a monotherapy report lower induction times (2-3 minutes) 29,34. Currently, the tendency is to begin with an initial bolus of 0.5-1 mg/kg and therefore it is likely that the induction time would have been less in group A. In this sense, it could be argued that after administering midazolam, we should have waited 2-3 minutes for it to take effect, which would result in a prolonged induction time in group B. However, if we had waited 2-3 minutes after administering the placebo in one group and midazolam in the other, the blinded nature of the study would have been jeopardized, as the endoscopist would have been aware of the greater sedation level of patients in group B before the second bolus was administered. In any case, the time elapsed between the initiation of sedation to the introduction of the endoscope (4 or 3 minutes in each group A and B, respectively) was sufficient for midazolam to begin to take effect.
Although the recovery time for the patient was shorter using propofol alone, the differences were not significant. Other studies have indeed described a synergic reaction when combining propofol with midazolam. Thus prolonging the patients´ recovery time significantly (30-33). In this sense, the study carried out by Levitzky et al. demonstrates the substantial advantage of BPS over traditional sedatives 27).
The quality of an endoscopic evaluation is essential in order to provide a precise diagnosis. Along these lines, we concluded that there were no differences between the two regimens with regard to achieving an adequate endoscopic visualization associated with the tolerance of insufflations. Meining et al. 13 used an identical scale to compare the administration of propofol in one arm and midazolam in the other. This study concluded that sedation with propofol greatly improved the quality of the endoscopy, while midazolam caused more belching, retching and a lower tolerance of inflation of the stomach.
Patient satisfaction constitutes an important advantage to the medication. There is no validated survey with regard to post-endoscopy patient satisfaction. The mGHAA-9 has been used to evaluate subjective aspects of endoscopy centers and hospital systems, although it is considered insufficient to evaluate patient satisfaction with the sedation 37,38. Patient satisfaction was similar in both groups of our study. Therefore, we may conclude that the combined use of propofol plus midazolam does not reduce patient satisfaction. Some studies have demonstrated a higher level of satisfaction in regimens that include propofol sedation 27,30,34. Amnesia is a highly desired effect for endoscopists. Both midazolam and propofol have amnesic effects, although the latter to a lesser degree 36. Memories of the procedure was slightly lower in the BPS group, which was not statistically significant. However, this reflects the results obtained by Molina-Infante et al. 30, which compared monotherapy with propofol to propofol plus midazolam.
Our study does have some limitations. Only ASA I and II patients under 80 years of age were included in the study. Other studies, have included higher risk and older patients and obtained similar results in terms of safety 6,12,30,31. Furthermore, this trial was designed to maintain a moderate sedation level and the exact level of sedation was not measured using the observer's assessment of alertness/sedation scale [OASS] 14. Therefore, we cannot discard the possibility of deep sedation at some point during the procedure. This is particularly true for group A, as BPS allows a more moderate sedation than propofol as a monotherapy 18,29.
Furthermore, the use of patient satisfaction surveys immediately after the procedure has been proven to overestimate the perceived satisfaction. Even though it is the simplest and perhaps the cheapest method which ensures patient response, satisfaction may decrease when surveys are filled out days after the endoscopic procedure. In this sense, the study of Lin et al. 39 compared both scenarios and concluded that despite the potential weakness of pre-discharge surveys, the magnitude of the differences between them was relatively small. In fact, there were significant values in only four of eleven questions of the survey. The survey used in our study was anonymous, as a non-anonymous survey might lead patients to evaluate the sedation more positively. This may help to diminish the differences found in the aforementioned study. In addition, the time at which the satisfaction survey was performed to compare the two sedation protocols was the same, before discharge from the hospital. Therefore, even though the survey may be biased to be more positive, there should be no bias when comparing both groups. Another issue might be the possible state of euphoria caused by the benzodiazepines, which may cause a higher score in the level of satisfaction in group B.
In conclusion, despite these limitations, the midazolam combined with propofol sedation regimen used in our study provided the same benefits of monotherapy with propofol without increasing adverse effects. This protocol offers a rapid sedation and recovery with a similar quality of the exploration and patient satisfaction, enhancing the existing body of evidence, which demonstrates the safety of endoscopic sedation supervised by endoscopists.
ACKNOWLEDGEMENTS
We would like to thank all the colleagues in the endoscopy unit who made this study possible.
REFERENCES
1. Igea F, Casellas JA, Gonzalez-Huix F, et al. Sedation for gastrointestinal endoscopy. Clinical practice guidelines of the Sociedad Española de Endoscopia Digestiva. Rev Esp Enferm Dig 2014;106:195-211. [ Links ]
2. Dumonceau JM, Riphaus A, Aparicio JR, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: non-anaesthesiologist administration of propofol for GI endoscopy. Eu J Anaesthesiol 2010;27:1016-30. DOI: 10.1097/EJA.0b013e32834136bf [ Links ]
3. Lazzaroni M, Bianchi-Porro G. Premedication, preparation, and surveillance. Endoscopy 1999;31:2-8. DOI: 10.1055/s-1999-13642 [ Links ]
4. Froehlich F, Gonvers JJ, Fried M. Conscious sedation, clinically relevant complications and monitoring of endoscopy: results of a nationwide survey in Switzerland. Endoscopy 1994;26:231-4. DOI: 10.1055/s-2007-1008949 [ Links ]
5. Tohda G, Higashi S, Wakahara S, et al. Propofol sedation during endoscopic procedures: safe and effective administration by registered nurses supervised by endoscopists. Endoscopy 2006;38:360-7. DOI: 10.1055/s-2005-921192 [ Links ]
6. Saenz-Lopez S, Rodriguez Muñoz S, Rodriguez-Alcalde D, et al. Endoscopist controlled administration of propofol: an effective and safe method of sedation in endoscopic procedures. Rev Esp Enferm Dig 2006;98:25-35. DOI: 10.4321/S1130-01082006000100004 [ Links ]
7. Sipe BW, Scheidler M, Baluyut A, et al. A prospective safety study of a low-dose propofol sedation protocol for colonoscopy. Clin Gastroenterol Hepatol 2007;5:563-6. DOI: 10.1016/j.cgh.2007.01.013 [ Links ]
8. Martínez J, Casellas JA, Aparicio JR, et al. Safety of propofol administration by the staff of a gastrointestinal endoscopy unit. Gastroenterol Hepatol 2007;30(3):105-9. [ Links ]
9. Morse JW, Fowler SA, Morse AL. Endoscopist-administered propofol: a retrospective safety study. Can J Gastroenterol 2008;22:617-20. DOI: 10.1155/2008/265465 [ Links ]
10. Lucendo AJ, Olveira A, Friginal-Ruiz AB, et al. Nonanesthesiologist-administered propofol sedation for colonoscopy is safe and effective: a prospective Spanish study over 1000 consecutive exams. Eur J of Gastroenterol and Hepatol 2012;24(7):787-92. DOI: 10.1097/MEG.0b013e328353fcbc [ Links ]
11. Friedrich K, Stremmel W, Sieg A. Endoscopist-administered propofol sedation is safe a prospective evaluation of 10,000 patients in an outpatient practice. J Gastrointestin Liver Dis 2012;21(3):259-63. [ Links ]
12. Sieg A, Beck S, Scholl SG, et al. Safety analysis of endoscopist-directed propofol sedation: A prospective, national multicenter study of 24441 patients in German outpatient practices. J Gastroenterol Hepatol 2014;29:517-23. DOI: 10.1111/jgh.12458 [ Links ]
13. Meining A, Semmler V, Kassem AM, et al. The effect of sedation on the quality of upper gastrointestinal endoscopy: an investigator-blinded, randomized study comparing propofol with midazolam. Endoscopy 2007;39(4):345-9. DOI: 10.1055/s-2006-945195 [ Links ]
14. Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol 1990;10(4):244-51. DOI: 10.1097/00004714-199008000-00003 [ Links ]
15. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth 1995;7:89-91. DOI: 10.1016/0952-8180(94)00001-K [ Links ]
16. Lightdale JR, Valim C, Newburg AR et al. Efficiency of propofol versus midazolam and fentanyl sedation at a pediatric teaching hospital: a prospective study. Gastrointest Endosc 2008;67:1067-75. DOI: 10.1016/j.gie.2007.11.038 [ Links ]
17. Thornley P, Al Beshir M, Gregor J, et al. Efficiency and patient experience with propofol vs conventional sedation: A prospective study. World J Gastrointest Endosc 2016;8(4):232-8. DOI: 10.4253/wjge.v8.i4.232 [ Links ]
18. Vargo JJ, Zuccaro G, Dumot JA, et al. Gastroenterologist-administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology 2002;123:8-16. DOI: 10.1053/gast.2002.34232 [ Links ]
19. Qadeer MA, Vargo JJ, Khandwala F, et al. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol 2005;3:1049-56. DOI: 10.1016/S1542-3565(05)00742-1 [ Links ]
20. Sethi S, Wadhwa V, Thaker A, et al. Propofol versus traditional sedative agents for advanced endoscopic procedures: a meta-analysis. Dig Endosc 2014;26:515-24. DOI: 10.1111/den.12219 [ Links ]
21. Wehrmann T, Kokabpick S, Lembcke B, et al Efficacy and safety of intravenous propofol sedation during routine ERCP. A prospective, controlled study. Gastrointest Endosc 1999;49:677-83. DOI: 10.1016/S0016-5107(99)70281-6 [ Links ]
22. Scott RPF, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia 1988;43:492-4. DOI: 10.1111/j.1365-2044.1988.tb06641.x [ Links ]
23. Hofmann C, Kiesslich R, Brackertz A, et al. Propofol for sedation in gastroscopy--a randomized comparison with midazolam. Z Gastroenterol 1999;37(7):589-95. [ Links ]
24. Eun Hye K, Sang Kil L. Endoscopist-Directed Propofol: Pros and Cons. Clinical Endoscopy 2014;47:129-34. DOI: 10.5946/ce.2014.47.2.129 [ Links ]
25. Short TG, Chui PT. Propofol and midazolam act synergistically in combination. Br J Anaesth 1991;67:539-45. DOI: 10.1093/bja/67.5.539 [ Links ]
26. McClune S, McKay AC, Wright PMC, et al. Synergistic interaction between midazolam and propofol. Br J Anaesth 1992;69:240-5. DOI: 10.1093/bja/69.3.240 [ Links ]
27. Levitzky BE, Lopez R, Dumot JA, et al. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone: a randomized clinical trial. Endoscopy 2012;44:13-20. DOI: 10.1055/s-0031-1291421 [ Links ]
28. Repici A, Pagano N, Hassan C, et al. Balanced propofol sedation administered by nonanesthesiologists: the first Italian experience. World J Gastroenterol 2011;17:3818-23. DOI: 10.3748/wjg.v17.i33.3818 [ Links ]
29. VanNatta ME, Rex DK. Propofol alone titrated to deep sedation versus propofol in combination with opioids and/or benzodiazepines and titrated to moderate sedation for colonoscopy. Am J Gastroenterol 2006;101:2209-17. DOI: 10.1111/j.1572-0241.2006.00760.x [ Links ]
30. Molina-Infante J, Dueñas-Sadornil C, Mateos-Rodriguez JM, et al. Nonanesthesiologist-administered propofol versus midazolam and propofol, titrated to moderate sedation, for colonoscopy: a randomized controlled trial. Dig Dis Sci 2012;57(9):2385-93. DOI: 10.1007/s10620-012-2222-4 [ Links ]
31. Seifert H, Schmitt TH, Gultekin T, et al. Sedation with propofol plus midazolam versus propofol alone for interventional endoscopic procedures: a prospective, randomized study. Aliment Pharmacol Ther 2000;14:1207-14. DOI: 10.1046/j.1365-2036.2000.00787.x [ Links ]
32. Chun SY, Kim KO, Park DS, et al. Safety and Efficacy of Deep Sedation with Propofol Alone or Combined with Midazolam Administrated by Nonanesthesiologist for Gastric Endoscopic Submucosal Dissection. Gut Liver 2012;6:464-70. DOI: 10.5009/gnl.2012.6.4.464 [ Links ]
33. Lee TH, Lee CK, Park SH, et al. Balanced propofol sedation versus propofol monosedation in therapeutic pancreaticobiliary endoscopic procedures. Dig Dis Sci 2012;57:2113-21. DOI: 10.1007/s10620-012-2234-0 [ Links ]
34. Sipe BW, Rex DK, Latinovich D, et al. Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc 2002;55:815-25. DOI: 10.1067/mge.2002.124636 [ Links ]
35. Külling D, Rothenbühler R, Inauen W. Safety of nonanesthetist sedation with propofol for outpatient colonoscopy and esophagogastroduodenoscopy. Endoscopy 2003;35(8):679-82. [ Links ]
36. Cohen LB, Hightower CD, Wood DA, et al. Moderate level sedation during endoscopy:?a prospective study using low-dose propofol, meperidine/fentanyl, and midazolam. Gastrointest Endosc 2004;59:795-803. DOI: 10.1016/S0016-5107(04)00349-9 [ Links ]
37. Loftus R, Nugent Z, Graff LA, et al. Patient satisfaction with the endoscopy experience and willingness to return in a central Canadian health region. Can J Gastroenterol 2013;27:259-66. DOI: 10.1155/2013/615206 [ Links ]
38. Ko HH, Zhang H, Telford JJ, et al. Factors influencing patient satisfaction when undergoing endoscopic procedures. Gastrointest Endosc 2009;69:883-91. DOI: 10.1016/j.gie.2008.06.024 [ Links ]
39. Lin OS, Schembre DB, Ayub K, et al. Patient satisfaction scores for endoscopic procedures: impact of a survey-collection method. Gastrointest Endosc 2007;65:775-81. DOI: 10.1016/j.gie.2006.11.032 [ Links ]
Received: October 06, 2017; Accepted: March 13, 2018











 texto en
texto en