INTRODUCTION
Proton pump inhibitors (PPI) comprise one of the most widely used pharmacological drug classes in the world. The PPIs currently marketed in Spain include omeprazole, pantoprazole, esomeprazole, lansoprazole and rabeprazole. Omeprazole is consumed the most, with 51.9 million packs consumed a year (5.5% of the total packs consumed). PPIs are the second most consumed group of drugs, accounting for 626 million Euros a year and a total of 4.8% of the prescription drug budget in Spain 1. PPI use has markedly increased over the last years, by more than 500% between 2000 and 2012 2, and Spain has a particularly high rate of use compared to other European Union countries 3,4,5,6.
According to the drug label of the Spanish Agency of Medicinal Products and Medical Devices' 7, PPI are approved for the following conditions: benign gastrointestinal ulcers, symptomatic gastroesophageal reflux disease (GERD), treatment of gastrointestinal ulcers due to non-steroidal anti-inflammatory drugs (NSAID) and prevention in at risk patients, and treatment and prevention of gastrointestinal ulcers due to Helicobacter pylori and Zollinger-Ellison syndrome. The following risk factors should be considered with regard to NSAID-induced ulcer prevention 3,5,6,8,9,10: age over 60 years, past history of ulcers or other complications, long courses of NSAID treatment at high doses, current concomitant therapy with other gastro-erosive drugs (other NSAID, anticoagulants, corticoids, selective serotonin reuptake inhibitors antidepressants) and severe comorbidities. In addition, PPIs are frequently used off label for conditions such as functional dyspepsia, uninvestigated dyspepsia and stress ulcer prevention in selected patients (critical condition, assisted ventilation, sepsis, severe burns and polytrauma) 3,5,6,8,9,10.
The rise in NSAID consumption and/or ageing of the population might explain the increase in PPI use, although alone they do not justify the remarkable rise over the last years. The most recent studies have been performed in both the inpatient and outpatient setting 11,12,13,14,15,16,17,18,19,20 and demonstrate the existence of PPI overuse. Furthermore, an important percentage of prescriptions (estimated at 25% in the European Union and 70% in the United States [3,18]) are not based on approved indications. A PPI use study was performed in La Princesa University Hospital, especially in the Emergency Department (ED), to assess the quantity and quality of PPI prescription.
OBJECTIVES
Primary objective
To evaluate the use of PPIs among patients treated at the ED of La Princesa University Hospital, as an indicator of both in-hospital and out-patient use.
Secondary objectives
Five aspects with regard to PPI prescriptions were analyzed:
If PPIs were correctly used as part of the patients' long term treatment regimen.
How PPIs were prescribed during the patients' stay in the ED.
How adequate was PPI prescription at hospital discharge.
Are there patients that have not been prescribed PPIs, even though they should?
Quantification of the appropriateness of the prescription.
MATERIALS AND METHODS
This was an observational, retrospective cross-sectional consumption and prescription-indication study performed at the ED of La Princesa University Hospital (Madrid, Spain). The sample size was calculated as 384 to obtain a confidence level of 95%, with a 5% margin of error and 50% heterogeneity. According to the ED personnel, the amount of patients seen daily in the ED ranged between 250 and 300, thus it was estimated that the necessary sample size could be achieved over two days (13th and 14th of January 2016). Patients older than 16 years of age who came to the ED and were attended by either medical or surgical emergency departments were included into the study. Exclusion criteria included patients that were transferred to the ED from the hospital outpatient clinic and patients with incomplete ED records. Pediatric and gynecological-obstetric patients were not included as our hospital does not have these departments.
Clinical data was revised and recorded in a Microsoft Office Excel 2010 database, which included the following: gender, age, relevant medical history, drug treatment, gastrointestinal risk factors, reason for consultation, clinical diagnosis, treatment during the stay in the ED, type of discharge (home or hospital), treatment at discharge and the Emergency Department responsible for prescription. The number of patients taking PPI before and after visiting the ED, whether it was indicated or not and if the dosage was correct were recorded.
Data were collected from the patients' electronic medical records, the patients did not need to be interviewed and informed consent was not necessary to obtain additional information. Thus, the study did not alter the ongoing clinical practice or the information stored in the electronic health system. In accordance to Spanish personal data protection laws, every patient was coded with a sequential number and no identifying information was recorded in order to preserve patient privacy. The study was approved by the local Ethics Committee for Clinical Research.
Approved indications and correct PPI dosage were defined in accordance with the PPI use optimization document by the Medical Assistance Coordination Commission of our center 8. This included: GERD (uninvestigated, non-erosive and erosive), dyspepsia (functional and uninvestigated), peptic ulcer, H. pylori eradication, NSAID-induced duodenal and/or gastric ulcer prophylaxis in at risk patients and stress ulcer prevention in at risk patients. This document considers omeprazole as the PPI of choice at a dose of 20 mg per day in all cases, except for severe erosive GERD and H. pylori eradication; the adequate dosage in these cases was considered as 20 mg twice a day 3,21. PPI use was also considered as adequate in patients that were undergoing gastric surgery, with medical literature that supports its use to prevent ulcers in this context 22,23.
Data analysis was performed using the IBMS SPSS Statistics program version 22. Pearson's Chi-square test was used for group comparisons and a statistical significance threshold of p < 0.05 was used. The following variables were analyzed: prevalence of PPI use, indication and appropriateness of prescribed PPI regime, the number of patients that should have been prescribed PPI but had not, and the appropriateness of the prescription.
RESULTS
The first 384 of 536 patients that were eligible and met all inclusion criteria were selected. Patients from the outpatient clinic were excluded as they were diagnosed and treated before going to the ED, and therefore, this group was not considered as comparable with the rest of the sample, who came to the ED directly from home.
Treatment before attending the ED
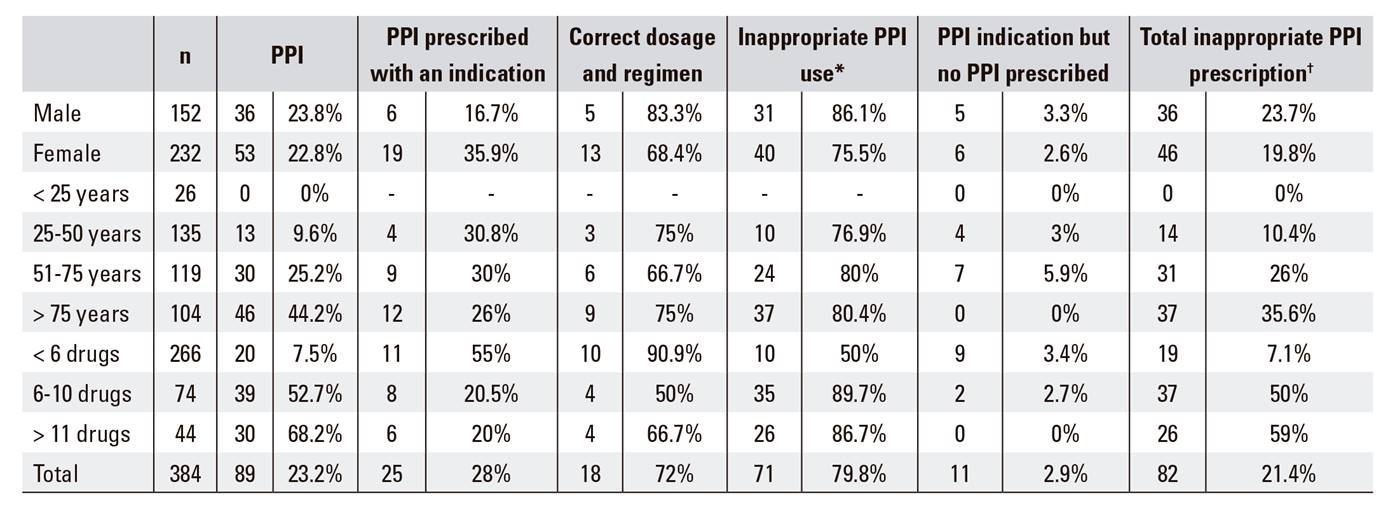
Two hundred and eighty-six patients (74.5%) were taking at least one drug before their visit to the ED and 23.2% (95% CI: 19%-26.9%) were taking a PPI (74.2% omeprazole, 19.1% pantoprazole, 4.5% lansoprazole and 2.2% esomeprazole). Only 28.1% of patients currently in treatment with a PPI had a clinical indication for its use and 72% had a correct regimen and dosage. Thus, 79.8% (95% CI: 75.7%-83.6%) of patients were taking a PPI inappropriately. Of the seven patients with inadequate scheduling and/or dosage, three had a higher dose, one had a lower dose, two had a higher frequency and one patient had both a larger dose and a higher frequency than necessary; 2.9% of patients with a clinical indication for PPI use (seven for GERD and four for NSAID ulcer prophylaxis) had not been prescribed PPIs. Thus, 21.4% (95% CI: 17.3%-25.2%) of the sample cohort had an inappropriate PPI prescription (PPI not indicated, indicated but with an incorrect regimen and/or dosage, or PPI was not prescribed). Indications for PPI use in the cohort were GERD in 61.1% of cases, NSAID induced ulcer prophylaxis in 22.2%, gastroduodenal ulcer treatment in 8.3%, 5.6% for post-gastric surgery ulcer prophylaxis and 2.8% for H. pylori eradication therapy. According to these data, 31.8% of patients with GERD and 50% of patients with NSAID ulcer prophylaxis were not receiving PPIs. The data with regard to the appropriateness of PPI consumption and prescription are summarized in Table 1. A significant (p < 0.001) increase in PPI use was observed both with age and the number of drugs, and a parallel increase in the overall lack of appropriate prescriptions was also observed (p < 0.001). PPI were significantly more frequently prescribed appropriately in females (p < 0.05) and males tended to have more correct prescriptions in terms of dosage and regimen. However, this did not reach statistical significance (p = 0.220). The total number of inappropriate PPI prescriptions was higher in males than in females, although this did not reach statistical significance (p = 0.367).
Treatment during ED admission
During their admission to the ED, 136 patients (35.4% of the total) had at least one pharmacological treatment prescribed, 12 (8.8%) of which received a PPI (eight omeprazole and four pantoprazole). Of the 12 patients treated with PPI, three (25%) were already taking a PPI as a part of their standard drug regimen. At discharge (50% discharged home and 50% hospitalized), PPI treatment was maintained (eight omeprazole, one pantoprazole and one esomeprazole) in ten patients (83.3%), although only six had a clinical indication (three dyspepsia, one gastroduodenal ulcer, one GERD and one NSAID ulcer prophylaxis). Among them, only one had an imperfect prescription, i.e., the dosage was higher than needed.
Treatment at discharge
The vast majority of patients (97.7%) were discharged with at least one prescription. The remaining patients did not have previous medications or were not prescribed pharmacological treatment at discharge. In addition, 30.5% (95% CI: 26.4%-34.3%) of patients received a prescription for PPI at discharge. This represents a significant increase in PPI prescription (p < 0.05) after the visit to the ED, compared with those who were taking a PPI beforehand (23.2%); 74.4% of patients discharged with a prescription for a PPI were already taking this medication before ED admission. PPI was withdrawn in two patients as it was not indicated. The distribution of prescribed PPI agents was very similar to the pre-hospital regimens (76.9% omeprazole, 18.8% pantoprazole, 2.6% esomeprazole, and 1.7% lansoprazole).
With regard to patients with a PPI prescription at discharge, only 33.3% had an indication, and of these, 84.6% had a correct dosage and regimen. This translates to 71.8% (95% CI: 67.7%-74.9%) of inadequate prescriptions at discharge. Of the six patients with PPIs correctly prescribed but with an incorrect dose/regimen, four had a higher dose than necessary, one had a lower dose than necessary and one had a higher frequency than necessary. Twenty-two patients (5.7% of the total sample) had a clinical indication for a PPI (seven GERD, 14 NSAID prophylaxis and one dyspepsia) but were not prescribed this medication upon discharge. Of the eleven patients with an indication for a PPI before attending the ED who were not taking it as part of their standard drug regimen, three were prescribed a PPI during the ED visit. Inappropriate PPI treatment was found in 27.6% of the sample cohort (95% CI: 23.6%-30.8%) at discharge, which is a significantly higher proportion (p < 0.05) compared to the level of inappropriate treatment before attending the ED.
PPI indications included 37.7% for NSAID ulcer prophylaxis, 36% for GERD, 8.2% for dyspepsia, 6.6% for gastroduodenal ulcer treatment, 6.6% for stress induced ulcer prevention, 3.3% for post gastric surgery ulcer prevention and 1.6% for H. pylori eradication. According to these data, 31.8% of patients with GERD, 60.9% with an indication for NSAID ulcer prophylaxis and 20% with dyspepsia did not receive a clinically indicated treatment with PPIs. Table 2 shows the data of PPI consumption and appropriateness. PPI consumption increases with age and the number of previous prescriptions (p < 0.001), with a consequent total increase in inappropriate prescriptions (p < 0.001). A difference between adequate prescriptions among male and female patients was noted, favoring the latter. However, this difference was not statistically significant (p = 0.081). Total inappropriate prescriptions by gender did not reach statistical significance, although there was a trend towards more inadequate prescriptions among males (p = 0.345).
Table 2 PPI use and appropriateness at discharge

PPI: proton pump inhibitors prescription. *PPI prescribed without an indication, or with an indication but with an incorrect dose and/or regimen. †Patients with inadequate PPI plus patients with an indication for PPI but not prescribed.
PPI by discharge type
The majority of patients (89.1%) were discharged home, while the remaining were admitted to hospital. The latter group received PPI in a greater proportion (71.4%) than the former (25.4%), which was statistically significant (p < 0.001). However, no differences were observed for indication (p = 0.369) or appropriateness (p = 0.287). The level of inappropriate prescriptions was similar between discharged (70.1%) and admitted patients (76.7%). Total PPI inappropriate prescriptions were higher (p < 0.001) among patients admitted to hospital (54.8%) compared to those discharged home (24.3%).
PPI by hospital department
The number of patients seen by each department, the type of department and PPI consumption and prescription appropriateness are represented in Table 3. Patients in medical departments received significantly more PPIs than in surgical departments (p < 0.001), and the latter had significantly more correct indications (p < 0.05). The total number of inappropriate prescriptions was significantly higher among medical departments (34.8%) than surgical departments (17.2%) (p < 0.001).
Table 3 PPI use and appropriateness broken down by hospital departments

PPI: proton pump inhibitors prescription. *Medical departments. **Surgical departments. ‡PPI prescribed without an indication, or with an indication but with an incorrect dose and/or regimen. §Patients with inadequate PPI plus patients with an indication for PPI but not prescribed.
DISCUSSION
Our results are in line with those of similar studies that demonstrate PPI overuse and inappropriate prescriptions, both in the inpatient and outpatient setting. The percentage of patients with a PPI prescription before visiting the ED (23.2%) was similar to patients seen in Primary Care 11,17 and inpatients admitted to the hospital 13,15,19; the prevalence of PPI prescription ranged from 18.6% to 34.6%. The proportion of patients admitted to the ED who were prescribed a PPI (71.4%) was similar to PPI usage studies among inpatients 12,13,14,15,18,19; the prevalence of PPI use ranges from 64.8% to 82.6%. However, other data such as PPI prescription within the ED (8.8%) and at discharge (25.4%) were not comparable, due to scarcity of studies on the topic 16. We think that our findings are of interest as they build upon previous studies that have analyzed PPI consumption. In addition, this study has the novelty of studying how PPIs are used in the ED, which is something that is infrequently reported in the literature. This study also considers inadequate prescriptions as those patients who should be receiving a PPI but were not prescribed this treatment, as well as those that were taking them without a clear clinical indication. This is an important point, as inappropriate prescriptions includes both over and under-prescription.
This study is limited by the fact that it is an observational, retrospective, cross-sectional study based on clinical electronic records from the ED. Thus, the information may be incomplete or inaccurate. Data such as previous medications or drugs given in the ED, or conditions and gastrointestinal risk factors that could warrant a PPI prescription may not have been recorded properly. These factors might have influenced our data with regard to the appropriateness of the prescription. The usage rate by PPI type was similar to that reported in other Spanish studies 11,12,17. Omeprazole was the most widely used, at around 75%, although the rate should approach 100% as it is the first choice agent based on local recommendations 8. In spite of this, pantoprazole is reported as the most widely used PPI in other countries such as the US 16. The most frequent indication for PPI use was GERD (both before and after the ED visit), followed by NSAID ulcer prophylaxis (higher after the ED visit), which is in contrast to other studies in which the predominant indication for PPI use was NSAID ulcer prophylaxis 11,12,13. In our study, there was a significant increase in both PPI usage and the rate of PPI inappropriate prescriptions as patient age increased, the former being a common finding in some studies 11,14,15,16,18,19, although this has not been reported in other studies 12,13,17. Prescription rates were similar among male and female patients, as other studies have also noted.
PPI use increased after discharge (from 23.2% to 30.5%) and this increase was more significant in hospitalized patients (71.4%) than in patients that were sent home (25.4%). The total PPI inappropriate prescription rate was higher among hospitalized patients (54.8%) than among discharged patients (24.3%). PPIs were not a commonly used drug during patient stay in the ED and only 8.8% of all ED patients received a PPI. Our usage rate was higher than that found in other studies, which have reported a usage of around 4.5% 16. That could mean that PPIs are not used for the symptomatic relief of gastrointestinal distress, NSAID ulcer prophylaxis is not considered until discharge, or perhaps their use is not reflected in the electronic health record.
With regard to the differences in prescription rates among different departments, medical departments prescribed more PPI than surgical departments, and this prescription was more inadequate. General services (the general ED and Internal Medicine) had a similar proportion of patients on PPI (clinically indicated), whereas there was a wider variation in other departments. Inappropriate usage and prescriptions were very high, although these data do not fully represent the prescription activity for PPI in many departments, as many patients were already taking PPI as a usual medication. This treatment is not reviewed during admission, or perhaps it is reviewed but medication errors are not seen or corrected. All of which demonstrates that the visit to the ED has been a lost opportunity to improve the patients' medication regime, as was suggested by Mazer-Amirshahi et al. 16.
Short courses of PPI treatment, such as those prescribed in the ED, have not been linked to adverse effects or drug interactions. However, initiating this treatment during the visit means it could be maintained indefinitely in the everyday medication of the patient 16 and tends to become indefinite if the indication is not reassessed 9,24,25. Thus, risks derived from PPI use arise. PPIs are, in most cases, safe and well tolerated (a factor that explains their widespread usage) but they are not harmless. Mild reactions such as headaches, nausea or abdominal pain have been reported, as well as some other less frequent but more serious events such as an increase in infections (pneumonia, C. difficile diarrhea), acid reflux, increased bone fracture risk, hypomagnesemia and acute interstitial nephritis. Furthermore, PPIs have the potential to interact with certain drugs by increasing gastric pH, for example, reducing the absorption of atazanavir or ketoconazole, which are weak bases, and increasing the absorption of weak acids such as digoxin, furosemide or acetylsalicylic acid. Furthermore, the inhibition of CYP2C19 (mostly omeprazole and esomeprazole) increases the effect of benzodiazepines such as diazepam or oral anticoagulants and lowers the effect of prodrugs such as clopidogrel. Given its safety profile, these side effects are often missed due to their very low frequency. However, PPI might put patients at risk, as with any other drug, so care should be taken with them 4,7,9,10,14,15,19,24,25,26,27.
The increase in PPI consumption and decreasing indication as age increases might translate to a belief that the elderly and/or polymedicated patients must be treated with PPI, and this could not be farther from the truth. In most cases, there is no benefit in combining PPIs with non gastroerosive drugs of pluripatology, as there are no risk factors. However, there is a potential for harm derived from the adverse effects and drug interactions with PPI use 13. This generalized overuse of PPIs and the high rate of inappropriate prescriptions demonstrates a widespread unfamiliarity with regard to their indications, or perhaps a lack of concern despite the existence and availability of recommendations for optimizing PPI use 8. Consequently, measures should be taken in order to achieve a better PPI use, such as improving the distribution of therapeutic recommendation guidelines. Studies have demonstrated its association with an improvement in the appropriateness of PPI prescription, paired with a decrease in interactions and adverse effects and a reduction in pharmaceutical spending 28,29. Medications should also be reviewed during every medical visit in order to detect and correct potential medication errors.
The appropriate use of drugs has not only become a concern for the medical community but also a matter of public interest, with patients being increasingly involved in their health. Recent initiatives driven by both healthcare professionals and patients, such as Choosing Wisely 16, promote a more responsible use of drugs and other healthcare resources by both users and providers. Interventions aimed at improving the appropriateness of prescriptions should not be limited to heath care personnel but should also include patients. The correct use of medication is everyone's responsibility.
CONCLUSIONS
The results obtained in this study demonstrate the existence of a very high PPI use and inappropriate prescriptions, both in the outpatient and the ED setting, which is in line with similar previous studies. The inadequate use of PPI occurs despite the existence of clinical guidelines with up to date information about the best prescription practices and points towards a lack of knowledge of their indications. The reconsideration of PPI prescription habits is mandatory. The prescription of PPI for approved and acknowledged indications at the minimum effective dose and reassessing the indication periodically are important steps to follow for a more responsible prescription. Due to these reasons, it is necessary to take bold steps in order to improve PPI usage, and thus limit possible drug interactions, undesirable side effects and unnecessary economic expenditure.