Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Cirugía Oral y Maxilofacial
versão On-line ISSN 2173-9161versão impressa ISSN 1130-0558
Rev Esp Cirug Oral y Maxilofac vol.27 no.5 Madrid Set./Out. 2005
Controversias en Cirugía Oral y Maxilofacial: Parte II
Delayed loading in implantology
Carga diferida en implantología
J.C. de Vicente Rodríguez
|
Abstract: Implant dentistry has become a scientifically based practice with the discovery of the biological basis of osseointegration. In the classical protocol, implants were load-free while bone was healing around them to ensure predictable osseointegration. However, several disadvantages of this approach have lead recently to a new protocol based on loading implants immediately after their placement. But, several questions about the outcome of this approach remain unanswered. Therefore, the aim of the present review article is to (i) review and analyze critically the biological basis of the load of implants, and (ii) propose guidelines for choosing the ideal moment to load dental implants, based on clinical variables. As a conclusion, delayed loading of implants remains the best protocol in several clinical situations. Key words: Dental implants; Osseointegration; Immediate loading; Delayed loading.
|
Resumen: La implantología dental se ha convertido en una práctica con bases científicas con el descubrimiento de la biología de la oseointegración. El protocolo terapéutico clásico implicaba mantener los implantes libres de carga hasta que estuviesen oseointegrados. Sin embargo, las desventajas de este procedimiento condujeron posteriormente al desarrollo de un nuevo protocolo, en el que los implantes son cargados de forma inmediata tras su colocación. Sin embargo, este nuevo abordaje suscita dudas acerca de su destino último que aún no han sido resueltas. El objetivo del presente artículo es (i) revisar y analizar críticamente las bases biológicas de la carga de los implantes y (ii) proponer guías orientativas para elegir el momento ideal de carga de los mismos, en función de variables clínicas. Como conclusión, se establece que si bien las cargas inmediata y diferida proporcionan los mismos resultados en diversas situaciones, la segunda es preferible en presencia de determinados factores de riesgo para el éxito de la primera. Palabras clave: Implantes dentales; Oseointegración; Carga inmediata; Carga diferida. |
Recibido: 08.06.2005
Aceptado: 22.06.2005
Jefe de Sección.
Servicio de Cirugía Oral y Maxilofacial. Hospital Universitario Central de
Asturias
(HUCA). Profesor Titular Vinculado. Facultad de Medicina,
Clínica Universitaria de Odontología, Oviedo, España.
Correspondencia:
Juan Carlos de Vicente Rodríguez
Calle Catedrático José Serrano s/n
33006 Oviedo, Asturias, España.
Telf.: 985 103 638
E-mail: jvicente@uniovi.es
Introduction
Edentulous mandibles, whatever the cause, generate such a degree of invalidity that rehabilitation measures have to be adopted. The traditional way of substituting missing teeth, in completely as well as in partially edentulous patients, has been by means of making a prosthesis that can be either removable, muco- or dento-muco-supported, or dento-supported, partially fixed prostheses according to the requirements and possibilities of each case. These therapeutic options have shown a growing preponderance over the last decades and they now represent, for most professionals, the first option in rehabilitation.
Following an unsteady start, and after the era of homoand xeno-dental transplants,1 the practice started of inserting into the jaw alloplastic devices made with a variety of materials and with a variety of imaginative designs2, 3 However, the scientific basis for implantology today was established in the 1960s decade and it has been attributed quite justly to Per-Ingvar Brånemark4, 5 who discovered, nearly by accident, the extraordinary biocompatibility of titanium and its resistance when attached to bone tissue. The term osseointegration was adopted to describe this relationship and it has since been solidly and permanently incorporated into medical terminology. Osseointegration implied the coexistence of an implant bearing masticatory loads with live bone tissue joined to its surface. This relationship between an artificial device and live tissue revolutionized treatment for oral invalidity. While the indications for implants and the surgical techniques used for placing them were at first limited, these have rapidly increased to numbers that at the onset of the technique were unthinkable, and there are now nearly a million cases treated by means of implantology. This is another example of a more general rule the more useful the technology, the faster the professional in charge of putting it into practice expand its limits, due to the user demands stimulating demands for refinements. One of the more obvious aspects of this scenario was when the implants were initially subjected to a prolonged healing stage that usually varied between three and six months. However, the growing demands of patients and the desires of oral surgeons and dentists that are not always impartial that are occasionally lacking scientific foundation, have shortened the time period between surgery and the placement of functioning prosthesis connected to the implants, to weeks, days or even hours or minutes. But, is this practice correct? Or, is it in fact responding to commercial criteria, putting these before basic biological principles with regard to healing and peri-implant bone remodeling? In order to answer these questions, we should have the biological basis behind osseointegration to back us. We will therefore describe in a succinct way how the osseointegration of implants is produced and the relationship with the different loading protocols.
Bone tissue and the bone-implant interphase
Bone is a specialized live connective tissue that is vascularized and dynamic, and made up of essential cells in a calcified extracellular matrix. This tissue has obviously been evolving for longer than the human species itself, and it is generally accepted that the origin of our cranium can be traced back to the appearance on the earth of protofish or cyclostomes6 at least 540 million years ago. As a result, current dental implants with their refined and advanced macroand micro- designs have "evolved" over a period of time that is incomparably shorter than that of the tissue supporting it that has two different macro-architectural forms and which has been combined, to a greater or lesser degree, into the different bones of the body: the trabecular or spongy bone and the cortical or compact bone. This subdivision has given rise to a clinical classification that distinguishes four "bone qualities"7 from Type I, with nearly all the maxillary bone being made up of this compact homogenous bone, to Type IV as, under a fine layer of cortical bone there is a core of low density spongy bone. This has been defined using purely clinical criteria, as "poor quality" bone, as the first implants with a machined surface often showed unsatisfactory results. However, from a biological point of view, this deficient clinical practice does not respond to bad bone quality as we will soon see.
At any moment of our lives, between 3 and 5% of our skeleton is being remodeled, that is to say, resorbed by osteoclasts and substituted by new bone produced by osteoblasts. This process of substitution by remodeling that begins at six weeks of intrauterine life and continues until the death of the individual, represents the biological basis that is responsible, under certain circumstances, for bone tissue being regenerated with tissue that is identical to the original, without any fibrous tissue repair, which represents the biological basis that permits the osseointegration of dental implants, irrespective of when these were loaded. This cellular component responsible for the formation of new bone is derived from the mesenchymal progenitors situated in the bone marrow that also provide the blood vessels needed for forming new bone, as well as the mononuclear precursors of the osteoclasts needed for tissue remodelation. As a result of this, bone trabeculae is remodeled faster than cortical bone, which from a biological perspective justifies the affirmation that in periimplant bone healing, trabecular bone is biologically better than the slow remodeling rate of the cortical bone. As a result it has been called "poor quality" bone (a paradox of "poor quality" bone)8 as opposed to the epithet given to it in the clinical classification previously mentioned. Fitting an implant implies the construction of a bed of bone sculpted with a drill with increasing sizes, which produces thermal and mechanical trauma of the receptor bed. If this trauma is moderate, the response by bone tissue will be gradual and it will involve the inflammation, reparation and remodeling phenomena. Initially, the well created by the osteotomy will be filled by a blood clot that will be removed when the implant is introduced. Following its insertion, the surface area of the device will adsorb biomolecules (fibrin, fibronectin, vitronectin, etc. through which bone forming cells will migrate and adhere to the surface of the implant. The osteoblasts synthesize bone matrix, which later calcifies encompassing the cells that formed it. These cells can deposit new bone on the surface of the implant or on preexisting neighboring bone according to the phenomena in contact and distance osteogenesis. 9 Initially, osseointegration was defined using the bone-implant interphase description with an optical microscope5, although it was viewed shortly afterwards from a clinical perspective, where an alloplastic implant would be secured by means of rigid fixation to the bone and this situation would hold over time in an asymptomatic fashion and in conditions of functional loading.10 Thus, loading the implants can be carried out at different times following installation. From the biological point of view this is irrelevant, if the process described is not interfered with of impeded in any way as, should this occur, instead of a bone-implant interphase there would be a union of the connective tissue and the implant. This was previously known by the euphemism "fibrointegration" as it was thought to imitate the periodontal ligament joining teeth to the alveolar bone. However, this connective tissue cannot be compared either structurally nor functionally with periodontal tissue, which is highly organized, with the result that this outcome is considered a therapeutic failure.
Stable internal fixation of bone fractures: A relevant precedent to immediate weight-bearing in implantology
Conventional treatment for fractures of the skeleton consists in immobilizing the member with the lesion (splint, plaster, traction, etc.) while ensuring that the fracture site is load free, until repaired. This conservative therapeutic paradigm has been shown to be efficient, although the patient has to pay a social and biological price (muscular atrophy, limitation of the range of articular motion, etc.) as a result of functional impotence due to bone continuity being broken. This treatment allows fractures to be repaired efficiently, if correctly reduced, although a certain deformity as a result of the fracture callus has to be expected. The principal limitation, as with delayed loading in implantology, is the belief that there is no other way of approaching the issue. The original idea that gave rise to a new way of treating fractures of the skeleton came from the Belgian surgeon Albin Lambotte, who in 190711 made the revolutionary statement that bone fractures could and should be allowed early mobilization - but in the absence of full weight-bearing as the materials used at that time in osteosynthesis lacked the necessary rigidity to allow this. However, who should also be recognized as the father of the biological principles behind internal fracture fixation is also a Belgian surgeon and a disciple of the first, Roberts Danis, who in 194912 established three basic principles for repairing fractures (i) the fractured bone should not be immobilized after the fracture, (ii) the treatment should restore the original shape of the bone according to Wolfs law and (iii) by means of a mechanism of "autogenous soldering", the bone fragments are joined with no visible callus. Following these studies, and in the mid 50s, Swiss National Security ordered a retrospective study that showed the existence of poor results with conservative fracture treatment. This led to 15 Swiss surgeons, headed by Maurice E. Müller (a disciple of Danis) to set up in 1958 the AO (Arbeitsgemeinschaft für Ostéosynthèsefragen) or ASIF (Association for the study of Internal Fixation), establishing their investigation center in the alpine city of Davos. The objective of the therapeutic revolution that they started consisted in the rapid recuperation of the form and function of the fractured bone, and for this four basic conditions were established: (i) Anatomic reduction of the displaced bone fragments following the fracture, (ii) stable fixation of the latter, (iii) preservation of the blood supply by means of an atraumatic surgical technique, (iv) active and pain-free early mobilization.13 From then on, a growing body of knowledge has been generated that has showed how fractured bones can bear weight immediately after surgery, providing there is stable internal fixation of the fracture site by means of osteosynthesis with plates and/or screws. In fact, early weight bearing of a fracture can be of advantage as it can help heal the fractured areas, as angiogenesis increases on bearing weight and the fracture site is actively remodeled. However, when conditions are not adequate the functional load of the fractured bone has to be delayed.
Weight-bearing time periods in implantology
As occurs with fractures of the skeleton, at the start of modern implantology it was deffended that following implant placement, the area operated on should be weight-free for 3- 6 months so as not to interfere with bone healing, and to encourage the osseointegration process.4, 14 The underlying reason behind this approach was that the micromovement of the implants as a result of functional loading could lead to the formation of fibrous tissue instead of bone, leading to clinical failure. In addition to this, they sought to cover the implants with soft tissue in order to prevent infection and the invasion of the bone-implant interface by epithelial tissue. However, this therapeutic modality has some inconveniences: (i) the patient has to avoid using a prosthesis for approximately two weeks following the surgery so that soft tissue healing is not interfered with, (ii) the experience is psychologically traumatic for many patients;15 (iii) during the healing stage there is a considerable functional limitation due to the transitory mobility of the prosthesis that is removable, and (iv) additional surgery is necessary and performed in a second stage.
As a result of the demands by patients on their surgeons the need arose to develop a routine protocol for implantology so that the delay during the healing period, before implant loading, was reduced or eliminated. The literature on the older and more recent loading protocols showed a variety of results that often could not be compared. Investigation methods were frequently inadequate and there was clear semantic confusion. With regard to this, there is a problem on analyzing the studies on immediate loading implants (IL), which is the absence of a single definition, and this leads to considerable confusion. Some refer to IL as being a period of a few hours, others however, refer to days after the placement of implants, while some even recommend loading these implants 3 weeks after placement. With the aim of clarifying this situation, it is necessary to distinguish between (i) immediate loading when this is carried out immediately after the placement of the implants (or at most within hours, but not days), which will avoid any possible blood clot disturbance during the important first stages of the healing process (ii) by early loading we understand that this is carried out days or weeks after the placement of implants but, in any event, before osseointegration has taken place. In reality, if this loading modality is opted for, it should be carried out after and not before the beginning of osteogenesis, as this increases with mechanical stimulation. As a result of this, early loading has to be carried out approximately 3 weeks after the healing period. (iii) We use the term conventional loading when the implants require a 3 to 6 months healing period before being loaded in a submerged or non-submerged way. This reflects the time needed for osteogenesis and the remodeling of woven bone into lamellar bone capable of weight bearing, according to the original recommendations of Brånemark y Schroeder. More recently, and as a result of the improved properties of the new implant surfaces, healing periods of 6 to 8 weeks are suggested. (iv) Finally, when the loading delay is more than this period, the term delayed loading is used. This occurs with implant placement when there is no primary stability, or when there is low bone density, or when bone-implant congruence is not good in post-extraction alveoli, or in bone regeneration procedures. The time-lapse between placing and loading the implants will vary between 6 and 12 months depending on the case.
It should be clarified that, irrespective of when the implants are loaded, the difference between the various protocols refers only to the initial phase of the treatment since, and as stated by Ganeles et al.,16 once there has been implant osseointegration there is no difference in long-term predictability of both protocols. Therefore, immediate loading is used by some professionals as in selected cases it has certain advantages over delayed loading: (i) masticatory function increases, (ii) the load transmitted to the implants through the mucosa covering them is reduced, (iii) there is better psychological tolerance to the treatment, and (iv) the duration of the latter is shortened.17 As a result, immediate loading can be a good therapeutic alternative to delayed loading, but only in selected cases. We will therefore review the situations where immediate loading and delayed loading have similar results, as well as others where delayed loading in preferable to immediate loading.
Studies on the efficiency of implant loading (delayed vs. immediate)
In a classic article Adell et al.14 studied the progress of 2.768 fixtures installed in 410 jaws of 371 completely edentulous patients. The implants, which were placed according to the submerged protocol, were loaded at 3 to 4 months in the mandible and later at 5 to 6 months in the maxilla. 81% of the maxillary implants remained stable during the 5 to 9 year observation period, with prosthesis stability being 89%. Over the same follow-up period, 91% of the mandibular fixtures remained stable while prosthesis stability was 100%. Nine years later, Adell el at18 reviewed the progress of 759 totally edentulous jaws in 700 patients with delayed loading implants, and they were followed for a maximum period of 24 years. In the maxillae more than 95% of patients showed prosthesis stability at 10 years and 92% at 15 years. Of the prostheses in the mandible, 99% were stable in all the time periods studied.
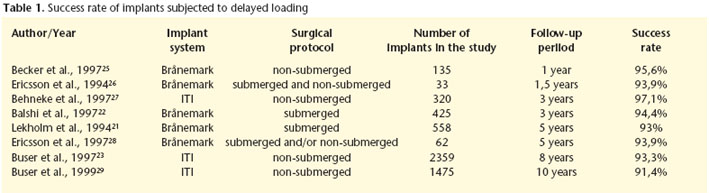
Noack et al.19 carried out a retrospective evaluation of 1964 implants in 883 patients that were followed for 16 years. The implants studied belonged to different systems (Brånemark, Frialit-1, Frialit-2, IMZ and Linkow blade implants). Of these 25.6% were placed in the maxilla and 74.4% in the mandible. The preprosthetic loss rate was 1.9% and 4.3% were lost after these were installed. The implants that were placed in the mandible showed a better survival rate than those placed in the maxilla (83 vs. 72% after ten years). Eliasson et al.20 examined the survival of implants used for supporting a complete-arch mandibular prosthesis. Brånemarks original concept established that these prostheses should be supported by six implants, although later evidence showed that four could be sufficient. Eliasson et al.20 rehabilitated the edentulous mandibles of 119 patients with complete-arch prostheses supported by four delayed loading implants. The survival rate of the implants after five years was 98.6%. Lekholm et al.21 carried out a prospective multicenter study in which 558 Brånemark implants were paced in 68 maxillae and in 91 mandibles, which were then followed for five years. All the implants were of the delayed loading type. Failure was defined using the following: implant mobility, persistent and incurable disturbance to peri-implant soft-tissue as well as mechanical problems affecting the anchorage unit, or bone loss reaching the implant apex third during the follow-up period. After five years the cumulative success rate was 92% in the maxilla and 94% in the mandible. Balshi et al.22 analyzed the influence of angulated abutments used for compensating the inclination of the implants with regard to implant survival. In order to do this they carried out a multicenter study of 71 patients that were fitted with implant-supported fixed prostheses, 63 were maxillary and 10 were mandibular. A total of 425 Brånemark implants were placed, 4 of which were lost before abutment connection. Of the remaining 421, 209 were connected to angular (experimental) abutments and 212 to standard (control) abutments. In all cases, the delayed loading protocol was used, with the second surgical stage taking place five months after the first intervention, or more, with the maxillary implants and three months or more with the mandibular implants. After the three-year follow-up period the following survival rates were observed. For the maxillary control implants 91.3%, for the maxillary test implants 94.8%. For the mandibular control implants, 97.4% and for the mandibular test implants 94.1% The differences observed were not statistically significant, and it was concluded that the use of angulated abutments in delayed loading protocols does not worsen the prognosis of dental implants. The prosthetic success rates were 96.8% for maxillary rehabilitation and 100% for mandibular rehabilitation.
Delayed loading implants can be placed in two surgical stages (fixation insertion followed by a second intervention for the transepithelial abutment connection) or in just one. A clear example of this last philosophy was adopted by the ITI implants. Buser et al.23 carried out a multicenter prospective evaluation of the success of non-submerged implants. For this they inserted 2.359 implants in 1003 patients consecutively and after a period of 3 to 6 months they were loaded with 393 removable prostheses and 758 with fixed prostheses. The check-ups were carried out annually for a maximum of eight years. During the healing period 13 implants were lost (early failure was 0.55%). Including the lost cases during the final following, the accumulated survival and success rates at 8 years was 96.7 and 93.3% respectively. As a reference in order to compare this with other studies having shorter follow-up periods, the survival and success rates at five years were 97.9 and 96.6% respectively. Levine et al.24 evaluated the use of ITI delayed loading implants that were used for single restorations. Twelve clinics in EEUU installed 174 implants in 129 patients and survival rates of 97.7% were observed after a 6-month follow- up period.
Table 1 shows the various success rates of delayed loading implants.
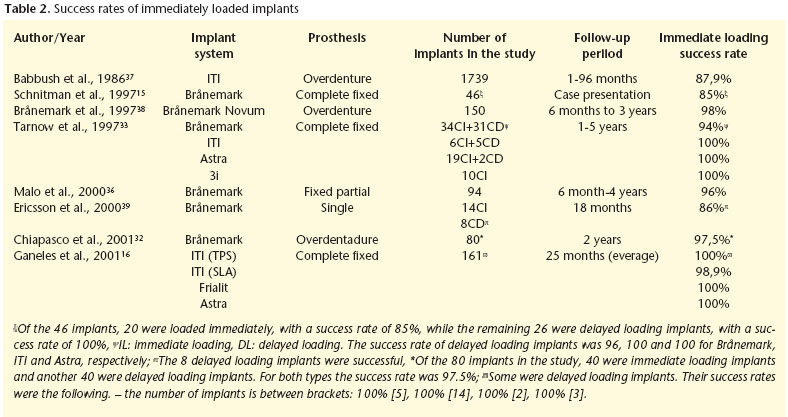
As with delayed loading implants, immediate loading implants have had the same prosthetic designs: overdentures, complete prostheses and partial implant-supported prostheses. Chiapasco et al.30 carried out a multicenter study of 226 patients with an average following of 6.4 years (ranging from 2 to 13 years) that were fitted with 904 implants in the intraforaminal area of the mental symphysis (4 implants per patient) that were loaded immediately. Of the 226 patients, 32 did not complete the study and the failure rate was 3.1%. Gatti et al.31 evaluated the results of 4 implant-retained overdentures with TPS surfaces that were immediately loaded. In 19 patients that were followed for 25 months, the accumulated survival rate was 96%. Later Chiapasco et al.32 compared the success rate of the implants loaded, some were immediate and some others were of the delayed type in 20 patients that were rehabilitated with mandibular overdentures. The success rate in both groups was 97.5%.
Tarnow et al.33 followed the progress of 69 immediately loaded implants and of another 38 submerged implants with delayed loading, that were all used to support fixed prostheses. Successful osseointegration took place in nearly 97% of the implants (104 out of 107). One of the submerged implants failed due to infection dissemination from the alveolus of a neighboring tooth that had been removed. On the other hand, two implants that had been loaded immediately were lost on removing a provisional prosthesis that had been cemented in order to study healing. An interesting result of this work is that differences between implants placed in the maxilla and in the mandible were not observed. Randow et al.34 compared the restoration of lower edentulous maxillae with implant-supported prostheses using two protocols, one with immediate loading and the other in two stage delayed loading. In the second group, the patients did not use the prostheses for ten days. After 18 months no differences were found between the two groups with implant survival being 100% in both groups. Horiuchi et al.35 placed immediate loading prostheses in both jaws - 10-12 Brånemark implants - and they observed a survival rate of 98% in the mandible and 96% in the maxilla after a follow-up period of between 8 and 24 months. For the success of immediate loading implant, these authors made the following recommendations (i) implants should be bilaterally splinted and at least 5 should be placed in the mandible and 8 in the maxilla with an optimum distribution; (ii) the length of the implants should be at least 8.5 mm (wide platform) or 10 mm (regular platform), (iii) implants should have good primary stability, (iv) cantilevers should be avoided in provisional prostheses, and (v) these should not be removed during the healing period. If these conditions are not met, we should assume as a result, that delayed loading is preferable to immediate loading.
There have also been trials involving the immediate loading of unitary implants although in some publications this is more like immediate "restoration" than immediate loading. This is in order to avoid occlusal contact of the prostheses, even when there is maximum intercuspidation. Malo et al.,36 carried out a study of 49 patients involving immediate loading of 94 Brånemark implants. Of the 54 fixed prostheses, 31 were crowns, and an implant survival rate of 96% after 2 years of functional loading was observed. The promising results regarding the immediate loading of crowns should be differentiated from the implants supporting complete prostheses, as in the former neighboring teeth protect the implants from traumatic occlusal loads during the initial phases of the healing process, providing the restoration is properly checked.
Table 2 shows the success rates of immediate loading implants.
The results of the studies on loading times in implantology, of which those appearing in tables 1 and 2 are just an example, suggest that delayed and immediate loading can achieve the same results. However, this could be an illusion, and to see what really lies behind this, one should analyze methodically each work published, which is obviously beyond the scope of this study, as is outlining the factors conditioning the different loading times. Most of the studies published on immediate loading were carried out with edentulous patients with implants in the interforaminal region of the mandible. Placing implants in the posterior sectors is often avoided as bone quality is lower, the masticatory forces are stronger and the risk of implant failure is predictably greater. While it would be unjust to generalize, a large proportion of the studies on immediate loading have defects with regard to the methodology used, and their value is therefore reduced. What stands out is (i) the small sample size, (ii) insufficient follow-up, (iii) in some studies submerged and non-submerged implants are placed simultaneously. The second set is of the immediate or early loading type with a temporary prosthesis and, after a healing period, the submerged implants are exposed and joined to the previous ones by means of a prosthesis. This means that, as a result of the different loading conditions, both types of implants cannot be compared under equal conditions, and (iv) whether the implants should have occlusal contact on the same day of the surgical procedure, or shortly afterwards, is debatable (immediate functional loading) or if not (non-functional immediate loading). Under these two different perspectives, the failures in the first group may not occur in the second, despite the fact that those using the second option insist on referring to immediate loading.
Factors conditioning timing in dental implant loading
In the studies on immediate loading of implants, various factors have been identified that condition therapeutic success. 40 These can be divided into four categories: (1) Surgical: (i) primary stability, (ii) surgical technique, (2) Tissular (i) quality and quantity of bone, (ii) healing, (iii) remodeling, (3)Implantological (i) macrostructure, (ii) microstructure (iii) dimensions and (4) Occlusal: (i) forces and (ii) prosthetic design.
Functional loading of an implant requires it to remain rigid41 to the extent that, of the factors conditioning therapeutic success at the time of establishing the load, implant stability is the most important. The micromotion of the implant that is over 10042 or 150 µm43 during the healing period, leads to mesenchymal differentiation of the implantbone- interface into fibroblasts instead of osteoblasts, which leads to a fibrous encapsulation instead of the osseointegration of the fixture as occurs in unstable bone fractures (pseudoarthrosis). Therefore, if an implant is placed in spongy bone with little density and with poor initial stability, delayed loading should be used once osseointegration has taken place and the stability that was lacking on placement appears. However, if there is initial stability, immediate or delayed loading can be chosen.
In addition to bone "quality", a precise surgical technique is also a key factor for achieving initial stability and the osseointegration of the implants, as excessive surgical trauma and the resulting thermal lesion can lead to osteonecrosis and the subsequent fibrous encapsulation of the implant. The temperature reached during the preparation of the implant bed depends on various factors of which the following stand out (i) refrigeration during drilling as, if this is insufficient and a temperature of over 47º is reached for a minute, thermal necrosis of the bone takes place;44 (ii) the load applied with the drill during the ostectomy. It has been reported that an increase, that is independent of either the speed or of the load, raises bone temperature, while the simultaneous increase in the speed and the load allows a more efficient cut with no significant increase in temperature; 45 (iii) the volume of the prepared bone, (iv) depth of the osteotomy, (v) thickness of the cortical bone and (vi) design and cut of the burr. After preparing the bed with very sharp burs with irrigation, the fixture has to be inserted. Depending on the design and the density of the recipient bone, the implants can be fixed with or without contouring. The relevance of this step is conditioned as if there is considerable resistance on insertion, the implant will have+ to be placed with considerable pressure, which can lead to microfractures appearing in neighboring bone. These lesions heal according to a well-known chain of events, according to the following sequence: angiogenesis, migration of bone forming cells, formation of an osteoid matrix, lamellar bone deposits and finally secondary bone remodeling.46
As previously mentioned, an implant inserted into highdensity bone is more likely to have initial stability and a capacity for supporting loads. In a resonance frequency analysis Friberg et al.47 observed that implants showed the same stability at the time of placement as they did 3-4 months later, providing the placement was into dense bone, as is usually found in the mental interforaminal region (72% of the symphyseal regions of the mandible have a Type I or II bone quality48). However, low bone density represents a risk factor for the success of the implants, independent of the time that they are loaded. Jaffin y Berman49 carried out a retrospective evaluation of the success of 1054 implants that were placed in bone of different density. Of the implants placed in Type I-III bone, only 3% of the fixtures were lost, while of those placed in Type IV bone (10% of the total) 35% were lost. As a result of this, and due to its favorable mechanical properties, most of the studies related with immediate loading were carried out in the anterior part of the mandible, where dense bone is normally found. It is well known that the different bone densities need different healing times. The lower the density of the bone, the longer the healing time before loading the implants. Therefore, for poor density bones (Type IV and some Type II bones) delayed loading implants should be used.
At what point implants are loaded also depends on tissue healing capacity, to the extent that when this is impaired (osteoporosis, diabetes, hyperparathyroidism, smoking, radiotherapy etc.), following a protocol of delayed loading is preferable and, on some occasions, an extended healing period should be expected. Among the conditions that interfere with bone quality and repair, diabetes and osteoporosis are among the more frequent. While osteoporosis is considered a risk factor in implantology, there is nothing in the literature supporting this when patients are treated with adequate loading protocols. While osteoporosis patients should be viewed as risk cases with regard to immediate loading on a level with or similar to that of Type IV quality bone, Friberg et al.50 observed in osteoporotic patients, an implant survival rate of 97% after a follow-up of 3.3 years.
Another situation where bone healing has to meet a greater reparative demand is when implants are placed in alveoli immediately after an extraction. Chaushu et al.51 compared implants loaded immediately in post-extraction alveoli with others placed in alveolar ridges that had already healed. The survival rates were of 82 and 100% respectively. Therefore, in view of the results of this study, if implants are loaded immediately, one out of every five fails.
Implants with a screw shape have the best mechanical retention and, as a result, better primary stability than cylinder shaped implants. Therefore, the former are more suitable for immediate loading than the latter. If a rough surface is added to a threaded design, the characteristics mentioned previously would have greater bone-implant unity and an increase in resistance to shearing forces of approximately five times. Jaffin et al.52 observed that the implants with a machined surface that were immediately loaded had a success rate of 83% with regard to implants with a TPS/SLA surface, that had a 99% success rate. However, despite the advantages declared, numerous studies carried out in animals and humans have not shown differences in results when different surfaces have been compared. The reason behind this apparent paradox may be due to the studies in humans being centered mostly in the symphyseal area of the mandible, where dense bone is to be found. This suggests that in order to achieve adequate stability, dense bone and a threaded macrostructure are conditioning factors that are more relevant than surface characteristics. Another parameter that has been thoroughly studied and that is significant for implant success, is length. If other variables are the same (width, surface) for every 3 millimeters that the length of a cylindrical implant increases, the surface area increases by 20-30%. As a result the studies on immediate loading have shown a failure rate of 50% with implants measuring 10 millimeters or less,15 so in these cases delayed loading is preferable to immediate loading.
Most of the studies on immediate loading exclude patients with masticatory parafunctions. Balshi y Wolfinger53 placed 130 implants in 10 patients, 40 were immediately loading implants and another 90 were submerged and involved delayed loading. After a follow-up period of 18 months, the survival rates were 80% for immediate loading implants and 96% for the delayed loading implants. They also observed that 75% of the failures that occurred with the first group took place in patients with bruxism. While bruxism is therefore a risk factor in implantology, when present for whatever circumstance, delayed loading is preferable.
Therefore, following this revision that is representative of what has been published but not exhaustive, we can make the following conclusions.
Conclusions
With the exception of some studies that are correct in terms of methodology, most on the immediate loading of dental implants present short follow-up periods, and there are even series of cases that are merely anecdotic. Even so, from these it can be deduced that, under certain conditions, treating partially and totally edentulous patients with immediately loaded implants, achieves acceptable results that are often better than those achieved with delayed loading. Therefore, on many occasions the choice of immediate or delayed loading is a question of preference of the patient and surgeons. As a result, in selected cases, immediate loading emerges as a valid alternative to routine protocols.
However, until there is more information on the subject, delayed loading, that is to say osseointegrated implants, is preferable in the following situations.
1. Implants with suboptimal stability due to being placed into bone with poor density or with a deficient surgical technique (non-coaxial drilling, excessive countersinking etc.) or due to the use of smooth surface implants or with a roughness that is very poor, or properly placed in freshly extracted alveoli that are not suitable.
2. Patients with biological risk factors (badly managed diabetics, osteopathy, irradiated patients and smokers, etc.
3. Implants with reduced and/or short diameters (less than 8 mm with a standard diameter or 10 mm long with a wider platform).
4. Implants combined with bone regeneration techniques (membranes, grafts, bone substitutes or derivatives) and normally submerged.
5. Malocculsion patients or masticatory parafunctions where the implants can become overloaded.
References
1. Fauchard P. Le chirurgien dentiste ou traite des dents. Deuxième édition revue, Tome premier à Paris chez Pierre-Jean Mariette, 1728. [ Links ]
2. Jourdan M, Magiollo M. Le manuel de lart du dentiste. Nancy, 1807. [ Links ]
3. Formiggini MS. Dental prosthesis in edentulous mouth by means of direct intramaxillary fixation. Riv Ital Stomatol 1954;9:814-22. [ Links ]
4. Brånemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scandinavian J Plast Reconst Surg Suppl 1977;16:1- 132. [ Links ]
5. Brånemark PI, Zarb GA, Albrektsson T (eds). Tissu-Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago: Quintessence, 1985. [ Links ]
6. Romer AS. The vertebrate body. Philadelphia: WB Saunders Co., 2nd ed, 1956:37-8. [ Links ]
7. Lekholm U, Zarb GA. Selección y Preparación del Paciente. En: Brånemark/Zarb/Albrektsson (eds). Prótesis Tejido-integradas. La Oseointegración en la Odontología Clínica. Berlín: Quintessence Verlags-GmbH; 1987:199-209. [ Links ]
8. Davies JE. Understanding peri-implant endosseous healing. J Dent Educ 2003;67:932-49. [ Links ]
9. Osborn JF, Newesely H. Dynamic aspects of the implant-bone interface. En: Heimke G, ed. Dental Implants: materials and systems. Munich: Verlag; 1980: 111-23. [ Links ]
10. Zarb GA, Albrektsson T. Osseointegration: A requiem for the periodontal ligament? (editorial). Int J Per Rest Dent 1991;11:88-91. [ Links ]
11. Lambotte A. Ltraitement des fractures. Paris: Verlag Masson, 1907. [ Links ]
12. Danis R. Theorie et pratique de lostéosynthèses. Paris: Libraries de LÀcademie de Medicine, 1949. [ Links ]
13. Schilch T. Surgery, science and industry. A revolution in fracture care, 1950s-1990s. Hampshire: Palgrave Macmillan, 2002. [ Links ]
14. Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg 1981;10:387- 416. [ Links ]
15. Schnitman PA, Wöhrle PS, Rubenstein JE, Da Silva JD, Wand NH. Ten-year results for Brånemark implants immediately loaded with fixed prostheses at implant placement. Int J Oral Maxillofac Impl 1997;12 :495-503. [ Links ]
16. Ganeles J, Rosenberg MM, Holt RL, Reichman LH. Immediate loading of implants with fixed restorations in the completely edentulous mandible: report of 27 patients from a private practice. Int J Oral Maxillofac Impl 2001;16:418- 26. [ Links ]
17. Kinsel RP, Lamb RE. Development of gingival esthetics in the edentulous patient with immediate loaded, single-stage, implant-supported fixed prostheses: a clinical report. Int J Oral Maxillofac Implants 2000;15:736-43. [ Links ]
18. Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. A long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants 1990;5:347-59. [ Links ]
19. Noack N, Willer J, Hoffmann J. Long-term results after placement of dental implants: longitudinal study 0f 1,964 implants over 16 years. Int J Oral Maxillofac Implants 1999;14:748-55. [ Links ]
20. Eliasson A, Palmqvist S, Svenson B, Sondell K. Five-year results with fixed complete- arch mandibular prostheses supported by 4 implants. Int J Oral Maxillofac Implants 2000;15:505-10. [ Links ]
21. Lekholm U, van Steenberghe D, Herrmann I, Bolender C, Folmer T, Gunne J, Henry P, Higuchi K, Laney WR, Lindén U. Osseointegrated implants in the treatment of partially edentulous jaws: a prospective 5-year multicenter study. Int J Oral Maxillofac Implants 1994;9:627-35. [ Links ]
22. Balshi TJ, Ekfeldt A, Stenberg T, Vrielinck L. Three-year evaluation of Brånemark implants connected to angulated abutments. Int J Oral Maxillofac Implants 1997;12:52-8. [ Links ]
23. Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, Belser UC, Lang NP. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life tabler analysis of a prospective multi-center study with 2359 implants. Clin Oral Impl Res 1997;8:161-72. [ Links ]
24. Levine RA, Clem III DS, Wilson TG, Higginbottom F, Saunders SL. A multicenter retrospective analysis of the ITI implant system used for single-tooth replacements: Preliminary results at 6 or more months of loading. Int J Oral Maxillofac Implants 1997;12:237-42. [ Links ]
25. Becker W, Becker BE, Israelson H, Lucchini JP, Handelsman M, Ammons W, Rosenberg E, Rose L, Tucker LM, Lekholm U. One-step surgical placement of Brånemark implants. A prospective multicenter clinical study. Int J Oral Maxillofac Impl 1997;12:454-62. [ Links ]
26. Ericsson I, Randow K, Glantz P-O, Lindhe J, Nilner K. Clinical and radiographical features of submerged and nonsubmerged titanium implants. Clin Oral Implants Research 1994;5:185-9. [ Links ]
27. Behneke A, Behneke N, dHoedt B, Wagner W. Hard and soft tissue reactions to ITI screw implants: 3-year longitudinal results of a prospective study. Int J Oral Maxillofac Impl 1997;12:749-57. [ Links ]
28. Ericsson I, Randow K, Nilner K, Petersson A. Some clinical and radiographical features of submerged and nonsubmerged titanium implants. A 5-year follow-up study. Clin Oral Implants Research 1997;8:422-6. [ Links ]
29. Buser D, Mericske-Stern R, Dula K, Lang NP. Clinical experience with one-stage, non-submerged dental impl. Adv Dent Res 1999;13:153- 61. [ Links ]
30. Chiapasco M, Gatti C, Rossi E, Haefliger W, Markwalder TH. Implant-retained mandibular overdentures with immediate loading. A retrospective multicenter study on 226 consecutive cases. Clin Oral Implants Res 1997;8:48-57. [ Links ]
31. Gatti C, Haefliger W, Chiapasco M. Implant-related mandibular overdentures with immediate loading: a prospective study of ITI implants. Int J Oral Maxillofac Implants 2000;15:383-8. [ Links ]
32. Chiapasco M, Abati S, Romeo E, Vogel G. Implant-retained mandibular ovedentures with Brånemark System MKII implants: a prospective comparative study between delayed and immediate loading. Int J Oral Maxillofac Implants 2001;16:537-46. [ Links ]
33. Tarnow DP, Emtiaz S, Classi A. Immediate loading of threaded implants at stage I surgery in edentulous arches: ten consecutive case reports with 1- to 5-year data. Int J Oral Maxillofac Implants 1997;12:319- 24. [ Links ]
34. Randow K, Ericsson I, Nilner K, Petersson A, Glantz PO. Immediate functional loading of Brånemark dental implants. An 18-months clinical follow-up study. Clin Oral Implants Res 1999;10:8-15. [ Links ]
35. Horiuchi K, Uchida H, Yamamoto K, Sugimura M. Immediate loading of Brånemark system implants following placement in edentulous patients: a clinical report. Int J Oral Maxillofac Implants 2000;15:824-30. [ Links ]
36. Malo P, Rangert B, Dvarsater L. Immediate function of Brånemark implants in the esthetic zone: a retrospective clinical study with 6 months to 4 years of follow-up. Clin Implant Dent Rel Res 2000;2:138-46. [ Links ]
37. Babbush CA, Kent JN, Misiek DJ. Titanium plasma-sprayed (TPS) screw implants for the reconstruction of the edentulous mandible. J Oral Maxillofac Surg 1986;44:274-82. [ Links ]
38. Brånemark PI, Engstrand P, Ohmell LO, Grondahl K, Nilsson P, Hagberg K, Darle C, Lekholm U. Brånemark Novum: a new treatment concept for rehabilitation of the edentulous mandible. Preliminary results from a prospective clinical follow-up study. Clin Implant Dent Rel Res 1999;1:2-16. [ Links ]
39. Ericson I, Nilson H, Lindh T, Nilner K, Randow K. Immediate functional loading of Brånemark single tooth implants. An 18 months clinical pilot follow-up study. Clin Oral Implant Dent 2000;11:26-33. [ Links ]
40. Gapski R, Wang H-L, Mascarenhas P, Lang NP. Critical review of immediate implant loading. Clin Oral Impl Res 2003;14:515-27. [ Links ]
41. RobertsWE, Smith RK, Zilberman Y, Mozsary PG, Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod 1984;86:95-111. [ Links ]
42. Brunski JB. Avoid pitfalls of overloading and micromotion of intraosseous implants. Dent Implantol Update 1993;4:77-81. [ Links ]
43. Szmukler-Moncler S, Salama H, Reingewirtz Y, Dubruille JH. Timing of loading and effect of micromotion on bone-dental implant interface: review of experimental literature. J Biomed Mat Res 1998;43:192-203. [ Links ]
44. Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent 1983;50:101-7. [ Links ]
45. Brisman DL. The effect of speed, pressure, and time on bone temperature during the drilling of implant sites. Int J Oral Maxillofac Implants 1996;11:35-7. [ Links ]
46. Schenk R, Hunziker EB. Histologic and ultrastructural features of fracture healing. En: Brighton CT, Friedlander G, Lane JM, eds. Bone formation and repair. Rosemont: American Academy of Orthopaedic Surgeons 1994, pp:117-46. [ Links ]
47. Friberg B, Sennerby L, Linden B, Grondahl K, Lekholm U. Stability measurements of one-stage Brånemark implants during healing in mandibles. A clinical resonance frequency analysis study. Int J Oral Maxillofac Surg 1999;28:266-72. [ Links ]
48. Misch CE. Bone density: a key determinant for clinical success. En: CE Misch ed., Contemporary Inmplant Dentistry. Chicago: Mosby 1999; pp:109-18. [ Links ]
49. Jaffin RA, Berman CL. The excessive loss of Brånemark fixtures in type IV bone: a 5-year analysis. J Periodontol 1991;62:2-4. [ Links ]
50. Friberg B, Ekestubbe A, Mellstrom D, Sennerby L. Brånemark implants and osteoporosis: a clinical exploratory study. Clin Impl Dent Rel Res 2001;3:50-6. [ Links ]
51. Chaushu G, Chaushu S, Tzohar A, Dayan D. Immediate loading of single- tooth implants: immediate versus non-immediate implantation. A clinical report. Int J Oral Maxillofac Implants 2001;16:267-72. [ Links ]
52. Jaffin RA, Kumar A, Berman CL. Immediate loading of implants in partially and fully edentulous jaws: a series of 27 case reports. J Periodontol 2000;71:833-8. [ Links ]
53. Balshi TJ, Wolfinger GJ. Immediate loading of Brånemark implants in edentulous mandibles: a preliminary report. Impl Dent 1997;6:83-8. [ Links ]











 texto em
texto em