My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Cirugía Oral y Maxilofacial
On-line version ISSN 2173-9161Print version ISSN 1130-0558
Rev Esp Cirug Oral y Maxilofac vol.35 n.1 Madrid Jan./Mar. 2013
https://dx.doi.org/10.1016/j.maxilo.2012.02.003
ORIGINAL ARTICLE
The role of custom-made prosthesis for temporomandibular joint replacement
Papel de la prótesis hecha a medida para la sustitución de la articulación temporomandibular
Louis G. Mercuri
River Forest, IL, USA
Professor of Surgery (Retired), Loyola University Chicago USA
Clinical Consultant, TMJ Concepts, Ventura, California USA
E-mail address: lgm@tmjconcepts.com
ABSTRACT
Alloplastic temporomandibular joint replacement (TMJ TJR) presents unique problems due to the integral and multifaceted roles this joint plays within the stomatognathic system to establish and maintain appropriate mandibular function and form. The TMJ not only acts as a secondary mandibular growth center pre-puberty, but is also crucial in maintaining proper mastication, speech, airway support and deglutition. Further, these essential life functions place the TMJ under more cyclical loading and unloading than any other body joint over a lifetime. Therefore, when TMJ TJR is indicated the device chosen must be able to provide long-term mandibular function and form outcomes.
End-stage TMJ pathology accompanied by physiological function and anatomical form distortions dictates the need for replacement. Due to the complex nature of joint related masticatory muscle functional and anatomical associations, it is unreasonable to expect an autogenous reconstructed TMJ or an alloplastic replaced TMJ can be returned to "normal" pre-morbid function. Therefore, as is understood with any orthopaedic joint replacement, patient and surgeon must agree and accept that there will always be some functional disability involved with any reconstructed or replaced TMJ.
Further, in the multiply operated, anatomically distorted patients, chronic neuropathic centrally mediated pain will always be a major component of their disability. Therefore, it is imperative that surgeon and patient understand that the primary goal of any TMJ TJR is the restoration mandibular function and form and that any pain relief must be considered of only secondary benefit.
This paper will discuss the role of custom TMJ TJR devices have in the management of severe and debilitating TMJ disorders.
Key words: Temporomandibular joint replacement.
RESUMEN
La sustitución aloplástica de la articulación temporomandibular plantea problemas exclusivos debido al papel esencial y polifacético que esta articulación desempeña en el sistema estomatognático para establecer y mantener la función y forma mandibular apropiadas. La articulación temporomandibular no sólo actúa como un centro prepuberal del crecimiento mandibular secundario sino que también es decisiva en el mantenimiento de la masticación, el habla, soporte de las vías respiratorias y deglución apropiadas. Además, estas funciones vitales esenciales producen en la articulación una mayor carga y descarga cíclicas que en cualquier otra articulación corporal durante la vida. Por consiguiente, cuando está indicada una sustitución aloplástica de la articulación, el dispositivo elegido debe ser capaz de proporcionar desenlaces favorables de la funcionalidad y forma mandibular a largo plazo.
La patología terminal de la articulación, acompañada de distorsiones de la funcionalidad fisiológica y de la forma anatómica, dicta la necesidad de su sustitución. Debido a la naturaleza compleja de las asociaciones funcionales y anatómicas de la articulación relacionadas con los músculos de la masticación, no es razonable esperar que la reconstrucción autóloga de la articulación o una sustitución aloplástica puedan restablecer la función premórbida "normal". Por consiguiente, como se entiende con cualquier sustitución ortopédica de una articulación, el cirujano y el paciente deben estar de acuerdo (y aceptar) que, en la reconstrucción o sustitución de la articulación, siempre estará presente cierto grado de discapacidad funcional.
Por otra parte, en pacientes con una distorsión anatómica por múltiples intervenciones, el dolor neuropático crónico, mediado centralmente, siempre será un importante componente de su discapacidad. Por esta razón, es indispensable que el cirujano y el paciente entiendan que el objetivo primario de cualquier sustitución de la articulación es el restablecimiento de la funcionalidad y forma mandibular y que el alivio del dolor debe considerarse tan sólo un beneficio secundario.
En este artículo, se describirá el papel que desempeñan los dispositivos hechos a medida para la sustitución de la articulación temporomandibular en el manejo de las enfermedades graves y debilitantes de la articulación.
Palabras clave: Sustitución de la articulación temporomandibular.
Introduction
Prior to the 1980s, the primarily reasons for TMJ reconstruction were the management of ankylosis, developmental maxillofacial deformities, severe inflammatory joint disease, or reconstruction after ablative tumor surgery or trauma. Thereafter, along with the aforementioned form and function challenges, there arose a group of patients requiring TMJ replacement who had previously undergone multiple unsuccessful invasive TMJ surgical procedures.2
As more of these complex patients presented, many were left with anatomically distorted and functionless joints, the result of the material failure of interpositional Proplast - Teflon (Vitek, Houston, TX) and/or Silastic (Dow-Corning-Wright, Arlington, TX). Interested reconstructive surgeons began developing goals to achieve physiologically reasonable, biologically rational and technically achievable outcomes not just considering form and functional requirements.
Consequently, based on decades of orthopaedic joint replacement experience, the following goals for TMJ replacement were developed and accepted3:
(1) Improvement mandibular function and form
(2) Reduction of further suffering and disability
(3) Containment of excessive treatment and cost
(4) Prevention of further morbidity
Indications for TMJ TJR
The following indications for custom TMJ TJR were then established4:
Inflammatory arthritis involving the TMJ, not responsive to other modalities of treatment
Since inflammatory arthritis involves a local, synovially mediated, destructive systemic disease process and complete synovectomy is not possible, the orthopaedic literature opts for alloplastic total joint replacement because the outcomes are very predictable.5
TMJ TJR in inflammatory disease has been discussed at length.6-19 These authors all agree that when the mandibular condyle is extensively damaged, degenerated or missing, as often seen in inflammatory arthritic conditions, TMJ TJR is a safe and effective approach to achieving optimal functional, esthetic and symptomatic improvement outcomes. Freitas16 reported on 12 arthritic non-growing patients (24 joints) requiring TMJ TJR. Six were managed with autogenous bone grafts and six with custom TMJ prostheses. The authors reported that based on the criteria established for the study, the custom TMJ TJR patients had statistically significant better subjective and objective outcomes than did those reconstructed with autogenous bone. In light of these results and the fact that the alloplastic TMJ TJR avoided the need for another operative site avoided potential morbidity, decreased operating room time and allowed for simultaneous mandibular advancement with predictable long-term results, they concluded that custom TMJ TJR was superior to autogenous bone grafting in arthritic TMJ disease. Reports indicating the long-term stability of custom TMJ TJR in patients with low-inflammatory or high-inflammatory arthritic conditions followed.17-19
Recurrent fibrosis and/or bony ankylosis not responsive to other modalities of treatment
The traditional management of TMJ complete bony ankylosis and re-ankylosis has been gap arthroplasty with autogenous soft or hard tissue graft reconstruction, or alloplastic hemiarthroplasty replacement. While autogenous grafting techniques can provide mandibular form, mandibular function is typically be delayed. Autogenous graft mobility during healing will compromise the graft's incorporation into the host bone and soft tissue environment or compromise the graft's nascent blood supply.2 It has been demonstrated that early mandibular mobility leads to graft/host interface failure.20
For the patient with re-ankylosis, placing autogenous bone into an area where reactive or heterotopic bone is forming intuitively makes no sense. Orthopaedic surgeons opt for total alloplastic joint reconstruction in similar situations in other joints.21 Therefore, in light of biologic factors and the orthopaedic experience, TJR should be considered the management option of choice for TMJ ankylosis and re-ankylosis.2,4,8
Custom TMJ TJR device components are designed and manufactured for each specific case and clinical situation from a protocol CT scan generated stereolithographic model with reported mean dimensional accuracy of 97.9%.22 Therefore, in ankylosis or re-ankylosis cases, the surgeon must in a first stage procedure free the ankylosis by creating an appropriate gap (2-2.5cm); place an anatomical spacer to prevent the reformation of tissue and/or bone23; and place the patient in maxillomandibular fixation (MMF) to prevent movement of the spacer or change in occlusion before protocol CT scan is made. The device is then designed and manufactured over the next 6 weeks.
During a second stage procedure, the spacer is removed, custom TMJ TJR fossa and ramus components are fixated, autogenous abdominal fat graft is placed around the articulation,24,25 and the patient begins immediate active post-operative physical therapy.
Pearce et al.26 described the use of pre-operatively developed templates to obviate the 2 staged protocol described above. However, it remains the author's opinion that to achieve the benefit of longevity provided by the custom TMJ TJR device in ankylosis cases, the best component-to-bone interface of the components will be achieved and assured by using the 2 staged approach. The concern about maintaining MMF between stages is moot in ankylosis since these patients from the start could not open their mouths before the first stage of this procedure.
Failed tissue grafts (bone and soft tissue)
Autogenous tissue grafting success requires that the host site have a rich vascular bed. Unfortunately, the scar tissue always encountered in the multiply operated patient and many end-stage disease TMJ cases does not provide an environment conducive to the predictable outcomes for free, or even the occasional vascularized, autogenous tissue graft. Marx reported that capillaries can penetrate a maximum thickness of 180-220µ of tissue, whereas, scar tissue surrounding previously operated bone averages 440µ in thickness.2 This may account for the clinical observation that free autogenous tissue TMJ reconstructions using cartilage, costochondral and sternoclavicular grafts often fail in cases of multiply operated patients or those with extreme anatomical architectural discrepancies resulting from end-stage pathology.12,13 Therefore, as with the ankylosis and re-ankylosis cases, custom TMJ TJR should be considered in the management of cases where failed tissue grafts are encountered.
Failed alloplastic joint reconstruction
Due to the osteolysis that occurs around past failed and particulated TMJ alloplastic materials and the resultant host bone architectural discrepancies created, it is difficult to adapt and stably fixate autogenous tissue or stock TMJ TJR device components to the distorted anatomical host bone of either the temporal glenoid fossa or mandibular ramus.
Further, the foreign body giant cell reactions which accompany failed or failing devices provide a poor environment for successful outcomes with an autogenous graft. Henry and Wolford confirm this as they reported that custom TMJ TJR provided more consistently predictable outcomes than did reconstruction with autogenous tissue in such cases.27
Loss of vertical mandibular height and/or occlusal relationship due to bony resorption, trauma, developmental abnormalities, or pathologic lesions
Loss of posterior mandibular vertical dimension due to developmental abnormalities, pathology, or traumatic injury all result in inconsistencies in both mandibular function and form. The later manifested as either an anterior (bilateral) or lateral (unilateral) apertognathia. After proper diagnosis of the etiology, correction of these maxillomandibular form and functional disorders should be directed to the site of the pathology - the TMJ.28
Custom TMJ TJR rather than osteotomy or autogenous tissue reconstruction or stock TMJ TJR should be considered in light of these the nature of the pathology, the patient's prior local surgical history and the state of the host bone architecture in these complex cases. Westermark et al. reported successfully managing large, complex mandibular defects involving the TMJ using a patient-fitted (custom) TMJ TJR system.29
Relative contraindications to alloplastic TMJ TJR
The literature considers the following to be relative contraindications to alloplastic TMJ TJR4:
Age of the patient
Since alloplastic TMJ TJR devices themselves have no inherent growth potential, the benefits of their use in growing patients over autogenous tissue must be considered carefully before their utilization in such cases. However, recent literature suggests that further investigation into the use of custom TMJ TJR in the growing patient may be justified in cases of re-ankylosis following unpredictable growth or failed autogenous tissue reconstruction.30
Mental status and competency of the patient
Is the patient psychologically prepared to cope with the permanent loss of a body part with the understanding that revision and/or replacement surgery in the future may be required? Does the patient have unrealistic expectations of complete relief of pain and normal jaw function after TMJ TJR? Is the patient willing and/or able to do the post-implantation physical therapy required to obtain maximum functional benefit from the procedure? Many of the multiply operated, functionless TMJ patients require pre-replacement referral to psychological and/or pain, and/or drug addiction counseling programs for them to accept the limitations of TMJ TJR to resolve their chronic pain/drug dependence issues.
Uncontrolled systemic disease
As with any form of an alloplastic implant - dental, orthopaedic or TMJ - once the potentially compromising systemic disease process has been controlled and the risk/benefit ratio is defined for the individual patient, TMJ TJR can proceed and should be monitored closely.
Active infection at the implantation site
Introduction of any alloplastic device into an infected or contaminated area can lead to failure of the device to stabilize in the host environment, ultimately resulting in its failure and loss. While this is true of all alloplasts, it is of particular concern with implants that have a planned long-term function under loading, such as with dental implants and TMJ TJR devices.
Documented allergy to the materials that are used in the devices to be implanted
Documented allergy to the biomaterials commonly used to manufacture TMJ TJR devices - commercially pure titanium, titanium alloy, cobalt-chrome-molybdenum alloy, ultra-high molecular weight polyethylene - is rare. Although 12-15% of the population can be sensitive to the nickel alloy in cobalt-chrome-molybdenum TJR components, far fewer reports of such allergic reactions have been reported in the orthopaedic literature.21 Patients with documented allergy to the component metals should not be exposed to that material in any new implanted device.
Discussion
There are two types of alloplastic TMJ TJR devices available, stock or "off the shelf" devices which the surgeon must make fit and custom TMJ TJR devices which are made to fit.
In the only report in the refereed oral and maxillofacial surgery literature that compares stock and custom TMJ TJR systems, the authors concluded that patients implanted with the study custom TMJ TJR had statistically significant better outcomes in both the subjective and objective domains than did those implanted with the study stock TMJ TJR devices.31
Why use a custom TMJ TJR device?
Utilizing criteria established for successful TJR by orthopaedists, an argument can be developed for the superiority of custom TMJ TJR devices32:
The components of any TJR system must be stabile in situ from implantation
All implanted alloplastic devices, be they dental implants, orthopaedic or TMJ TJR devices, depend on the principle of osseointegration of the fixation components (screws, in the case of orthopaedic and TMJ TJR devices) for their ultimate stability and longevity. Osseointegration implies the direct incorporation of the fixation components with the host bone without the preliminary phase of fibrous tissue ingrowth.33 The requirements for osseointegration are essentially the same as for primary fracture healing - the transmission of forces from the implant to the bone and vice versa must occur without relative motion or without intermittent loading.33
The most important principle in TMJ TJR surgery must be the stability of the device components at implantation. In orthopaedic surgery, some TJR devices can be initially stabilized by press-fitting or cementation into the cancellous shaft of the host long bone. However, the anatomy of the TMJ itself, mandibular ramus and the temporal glenoid fossa do not allow those options. Therefore, initial fixation and stabilization of all present TMJ TJR device fossa and ramus/condyle components must be provided by screws (Fig. 1).
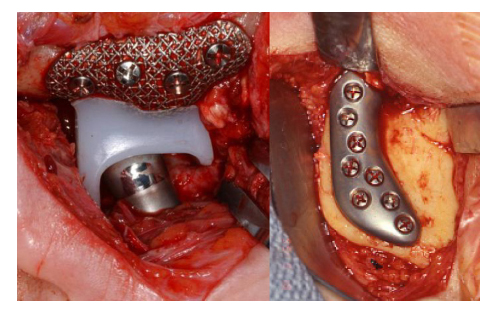
Fig. 1. Custom TMJ TJR fossa and ramal components screw fixated
(TMJ Concepts, Ventura, CA). Commercially pure titanium (CP Ti)
mesh fossa backing for UHMWPE articulating surface with posterior stop;
wrought alloyed titanium (Ti-6Al-4V) ramus component and wrought Co-Cr-Mb
articulating condylar head.
Compounding the stability and fixation issues is the fact that most patients presenting with indications for TMJ TJR have distorted anatomy, the result of either numerous failed prior surgical interventions, material or device failures, or by primary or secondary end-stage joint pathology (i.e., rheumatoid arthritis). This makes it extremely difficult to effect a long-term stable reconstruction with stock TMJ TJR device components.
All stock TMJ TJR devices require the surgeon to "make the components fit". To do this these devices commonly require precious host bone be removed at implantation, or often one or both components must be bent to fit, shimmed with bone or alloplastic cement in order to develop some reasonable component-to-host bone interface or "fit". Such maneuvers can lead to component material fatigue overload which may promote early material failure with functional loading.
More concerning is that any of these alterations that result in inadequate component-to-host bone contact can result in micromotion of the stock device components which may lead to suboptimal screw fixation osseointegration. Micromotion leads to the formation of a fibrous connective tissue interface between the altered TMJ TJR component and the host bone resulting in early loosening of the screw fixation. This can lead to component mobility and potential early catastrophic or certain future premature device failure (Fig. 2).

Fig. 2. Failed bilateral metal-on-metal stock TMJ TJR
device 1-year post-implantation.
Note poor adaptation of both ramal components,
loose screws and fractured left fossa.
Custom TMJ TJR devices are "made to fit". The components can be designed and manufactured to conform and manage any unique anatomical situation (fig. 3). At implantation, neither the components nor the host bone require alteration, augmentation or supplementation to achieve initial overall component-to-host bone stability maximizing the prospect for fixation screw osseointegration. This results in screw fixation that secures the custom TMJ TJR device components to the host bone, mitigating the potential for micromotion and is responsible for the long-term reported stability and function of custom TMJ replacement devices.34-37

Fig. 3. Custom TMJ TJR components (TMJ Concepts, Ventura CA)
demonstrating the variability of anatomical architecture resulting
in different component designs within the same patient.
The materials from which patient-fitted (custom) TMJ TJR devices are manufactured are biocompatible and able to withstand the forces of mandibular function
In 1960, Sir John Charnley reported the use of a total alloplastic prosthetic hip replacement system utilizing an ultra-high molecular weight polyethylene (UHMWPE) polymer acetabular cup articulating with a stainless steel femoral head component, both of which were cemented to place with polymethylmethacrylate.38 Modifications of this device utilizing titanium, titanium alloy and cobalt-chromium-molybdenum have become the gold standard for low friction orthopaedic TJR surgery to date.21
The custom TMJ TJR device with the most published studies in refereed literature is manufactured using commercially pure titanium (CP Ti) as the fossa backing for UHMWPE articulating surface; wrought alloyed titanium (Ti-6Al-4V) for the ramus component and wrought Co-Cr-Mb for the articulating condylar head (Fig. 1).
TJR devices must be designed to withstand the loads delivered over the full range of function for each patient's specific clinical and anatomical situation
The advantage a custom TMJ TJR device affords, besides stability of fit at implantation and composition materials, is that the device components are specifically designed to manage the loads posed in any clinical situation.
Stock TMJ TJR systems in which there are multiple "make fit" choices either thin cast Co-Cr fossa or all UHMWPE fossa components as well as cast Cr-Co ramus/condyle components can pose multiple pitfalls.Metal-on-metal geometry to be successful in orthopaedic TJR can only be applied to a TJR hip where tightly constrained radial clearances of less than 200µm between the metal acetabular cup and metal femoral head are mandatory or wear related metal particulation will lead to metalosis, osteolysis, loosening, and micromotion leading to device failure.39-42
Metal-on-metal devices would never be designed for knee TJR because of that joint's functional anatomy is not constrained as is the hip joint. The same should be true of the TMJ, whose functional anatomy is also not constrained, even after TJR. Stresses and strains directly or eccentrically vectored against an incomplete or inadequate component-to-bone interface or unstable thin cast Co-Cr fossa by the metal condylar head of a stock TMJ TJR system will lead to micromotion, component screw loosening and/or fossa component thin cast metal fatigue and fracture (Fig. 2).
Stock TMJ TJR devices in which a UHMWPE flange is utilized to fixate the fossa component with screws to the temporal bone and zygomatic arch offers the potential for problems that can lead to loosening of the stock fossa fixation screws, increased micromotion under load and eventual failure of the component and the device.
Hallab43 enumerated the reasons why an un-backed all-UHMWPE fossa component is not favored in orthopaedics, especially when placed against host bone:
1) Increased back-side wear (component-to-host bone) under function
2) Poor surface for bone fixation (hydrophobic UHMWPE vs. hydrophilic bone)
3) Decreased bone remodeling on surface of the UHMWPE
4) No macro-texturing to enhance short and long-term bone attachment strength
5) Can lead to increased potential for biofilm infection (due to decreased cell attachment)
6) Increased chance of "cold flow" and UHMWPE fracture.
7) Less control over host bone side implant orientation due to greater likelihood of osteolysis on the host bone side over time.
8) Poor surface for cementing and will likely results in high wear and micromotion.
Lastly, since all present stock and a metal-on-metal custom TMJ TJR fossa component do not have a posterior stop, the potential for posterior dislocation of the device condylar head exists. If not perfectly aligned in the center of the stock fossa both medio-lateral and antero-poterior, posterior displacement of these condylar heads can impinge on the auditory canal resulting in pain, malocclusion or infection should a pressure related perforation of the cartilaginous auditory canal occur. This should be of particular concern when using any TMJ TJR device that does not have a posterior fossa component stop for orthognathic cases while setting the condyle into "centric" during mandibular procedures, and especially in combination with counterclockwise mandibular rotation procedures (Fig. 4). Custom TMJ TJR fossa components are designed and manufactured with fossa component posterior stops to accommodate such situations.

Fig. 4. Lack of posterior fossa component stop resultingin
posterior displacement of condylar head of a stock metal-on-all UHM
WPE TMJ TJR device after bi-maxillary orthognathic surgery.
Outcome data from custom TMJ TJR devices reveal few device failures to date attributable to the inability of these devices to withstand standard loads when the appropriate geometry and materials are used.34-37 For example, the center of rotation of the condyle of a patient-fitted (custom) TMJ TJR device can be moved vertically to accommodate closure of the open bite deformity seen in the rheumatoid patient16-19; the ramus component can be shaped to accommodate the amount of host mandibular bone available. This ability to vary the design to deal with the available anatomy leads to a more predictable result under the expected loads delivered in each complex situation27 (Fig. 5).

Fig. 5. Custom TMJ TJR device (TMJ Concepts, Ventura CA)
designed to manage both fossa and mandibular defects after ablative surgery.
(Courtesy of Dr. GR Fisher, New Orleans, LA).
Custom TMJ TJR devices can be designed to provide maximum screw fixation for initial stability by avoiding the inferior alveolar neurovascular bundle thereby eliminating the potential damage to this structure during screw placement. Also, because the components are custom made, the proper screw length can be pre-determined and prescribed for the surgeon eliminating the time consuming and often frustrating intra-operative "screw hole probing" in an attempt to determine proper bi-cortical fixation screw lengths. More importantly, knowing the proper screw lengths prevents the use of screws that are too long, the medial tips of which can irritate the temporalis and/or the medial pterygoid muscle during mandibular function causing post-implantation complaints of post-implantation functional muscular pain.
The implantation surgery must be performed for the proper indications and aseptically
As with any management option, outcomes are only predictable when what is done is done for the right reason, at the right time, for the right patient, the right way, and with the right device.
Schmalzried and Brown39 report that the major causes of orthopaedic TJR failures are the result of surgical technique or limitations of the TJR device to be able to deal with the anatomical situation presented for replacement. The utilization of a patient-fitted (custom) TMJ TJR device would mitigate both issues.40
Questions and concerns
The following issues are often raised concerning custom TMJ TJR devices and require further clarification and discussion:
Custom TMJ TJR devices are expensive
First, TMJ TJR devices are thought to be more costly than autogenous tissue. Consider the extended surgical time, personnel and resources required to complete an autogenous TMJ reconstruction. Add to that the potential for increased morbidity associated with harvesting autogenous tissue and the increased length of hospital stay should donor site complications occur. Compare that to TMJ TJR for the same case. The cost of the later in time, personnel would be far less overall and since there is no secondary donor site surgery, the potential for such complications are negligible.
Secondly, custom TMJ TJR devices, since they are "made to fit," require less surgical time than do stock devices that the surgeon has to "make fit". Also, depending often on location and factors often out of the control of the manufacturer, custom TMJ TJR devices are generally equal or slightly less in price than stock devices.
Material wear and long-term stability and survivability of TMJ TJR devices
There is no argument that because TMJ TJR is a biomechanical rather than a biological solution to severe, end-stage TMJ pathology, future for revision surgery to remove scar tissue from the articulating components of the implant or even replacement of the implant over time due to material wear and/or failure may be required.40
However, material wear and long-term stability and survivability of custom TMJ TJR devices concerns are be mitigated by the use of proper biomaterials and design configurations to decrease material wear and increase device longevity under functional loading as described above.
Post-operative physical therapy issues
Since the components of a custom TMJ TJR device interface so well with the host bone and the fixation is stable from the time of implantation of the components, mandibular function can begin immediately after implantation. This is considered an essential component of rehabilitation in orthopaedic TJR because muscle function has been compromised over time in such cases. Salter44 in his work on Continuous Passive Motion (CPM) after orthopaedic joint surgery has shown the importance of this concept to the long-term functional results of joint surgery.
Potential adverse outcomes
Just as with any surgical procedure, adverse outcomes or complications may occur during or following implantation of any TMJ TJR device requiring further management. Complications may be related to or influenced by the patient's previous surgical history or prior medical conditions. The most common complications seen with custom TMJ TJR devices include but are not limited to:
Continued or increased pain levels or worsening of other present TMJ symptoms
It has been reported that as the number of prior TMJ surgeries increases, the lower the subjective outcomes improvement measures.27,34-37,45 However, objective outcomes and quality of life measures are reported improved in long-term follow-up.37 There is also a report of similar subjective, objective and quality of life findings in patients previously exposed to failed alloplastic materials (Proplast-Teflon and silicone rubber).36
Further, the presence of comorbid conditions in patients with temporomandibular joint disorders (TMD) also may explain why 50% of patients seeking care for TMD pain, some of whom were multiply operated and/or exposed to failed materials or devices, still report experiencing pain five years later, and 20% of patients experience long-term disability from chronic pain.1,46
Infection
Fortunately post-implantation TMJ TJR infections are rare. When they occur, they are typically superficial and are resolved simply using appropriate antibiotic and minor surgical management.47 However, the orthopaedic literature reports a 1-2% incidence of biofilm infections in orthopaedic implants.48,49 Management of biofilm infections of total joint devices involves removal and remake/repassivation of the device components, placement of an appropriate antibiotic spacer in the area of the device, long-term antibiotic management and reimplantation of the new device once all signs of infection have resolved.50
Levent et al. developed a prospective study to examine the significance of 5 variables commonly associated with the potential for surgical site infections (SSI) after knee TJR: (1) classic risk-factors (e.g. diabetes, rheumatoid disease); (2) incomplete pre-operative skin preparation; (3) Methicillin resistant Staphylococcus aureus (MRSA) positive patient; (4) peri-operative antibiotic usage; (5) duration of surgery in 364 consecutive patients. After a 1 year median follow-up, they report a 1.4% SSI rate and of the 5 variables only peri-operative antibiotic usage and duration of surgery demonstrated significance.51
Since custom TMJ TJR components are "made to fit" manipulation and implantation time will be less than stock TMJ TJR components that the surgeon must "make fit" thereby potentially lessening the potential for post implantation infection.
Heterotopic bone formation
Heterotopic bone formation is the presence of bone in soft tissue surrounding an alloplastic joint replacement where bone normally does not exist52 and leads to decreased joint mobility and pain. Imaging is used to distinguish it from other diagnostic possibilities. As treatment or prophylaxis, either a non-steroidal anti-inflammatory drug, such as indomethacin,53 a diphosphonate, such as ethane-1-hydroxy-1, 1-diphosphate,54 or local radiation therapy55 have recommended. Surgical resection is used to preserve joint mobility; however, heterotopic bone formation is likely to recur and possibly progress, therefore it is recommended that an autogenous fat graft be packed around the articulation of TMJ replacement devices to decrease this potential.24,25
Conclusion
The modern practice of orthopaedic surgery would be impossible without the availability of alloplastic TJR devices; therefore TMJ TJR devices should likewise have a definite place in the armamentarium of the oral and maxillofacial reconstructive surgeon for the management of the severely degenerated, anatomically distorted, functionless TMJ patient. As has been well documented in the orthopaedic and TMJ literature, the potential for an increase in the quality of life these patients gain post TJR is an important consideration.
Custom TMJ TJR devices, because of their design, materials from which they are manufactured, their inherent stability appear to provide improved long-term successful outcomes for patients with end-stage anatomical TMJ disease.
Conflict of interest
Dr. Mercuri was instrumental in the development of the TMJ Concepts TJR System, compensated as Clinical Consultant, and is a shareholder.
References
1. Velly AM, Look JO, Carlson C, et al. The effect of catastrophizing and depression on chronic pain - a prospective cohort study of temporomandibular muscle and joint pain disorders. Pain. 2011; 152:2377-83. [ Links ]
2. Mercuri LG. Alloplastic temporomandibular joint reconstruction. Oral Surg. 1998; 85:631. [ Links ]
3. Mercuri LG. The use of alloplastic prostheses for temporomandibular joint reconstruction. J Oral Maxillofac Surg. 2000; 58:70. [ Links ]
4. Mercuri LG. The TMJ Concepts patient fitted total temporomandibular joint reconstruction prosthesis. In: Donlon W.C., editors. Temporomandibular joint reconstruction. Philadelphia: Oral and Maxillofacial Surgery Clinics of North America, Saunders; 2000. [ Links ]
5. Hollingsworth J. Management of rheumatoid arthritis and its complications. Chicago: Yearbook Medical Publishers, Inc.; 1978. [ Links ]
6. McBride KL. Total temporomandibular joint reconstruction. In: Worthington P., Evans J.R., editors. Controversies in oral and maxillofacial surgery. Philadelphia: Saunders; 1994. [ Links ]
7. Kent JN, Misiek DJ. Controversies in disc condyle replacement for partial and total temporomandibular joint reconstruction. In: Worthington P., Evans J.R., editors. Controversies in oral and maxillofacial surgery. Philadelphia: Saunders; 1994. [ Links ]
8. Total temporomandibular Joint Reconstruction. Donlon W.C., editors. Oral and Maxillofacial Surgery Clinics of North America. vol. 12. Philadelphia: Saunders; 2000. [ Links ]
9. Stern NS, Trop RC, Balk P. Total temporomandibular joint replacement in a patient with rheumatoid arthritis: report of a case. JADA. 1986; 112:491. [ Links ]
10. Zide MF, Carlton DM, Kent JN. Rheumatoid disease and related arthropathies. I. Systemic findings, medical therapy, and peripheral joint surgery. Oral Surg. 1986; 61:119. [ Links ]
11. Kent JN, Carlton DM, Zide MF. Rheumatoid disease and related arthropathies. II. Surgical rehabilitation of the temporomandibular joint. Oral Surg. 1986; 61:423. [ Links ]
12. Speculand B, Hensher R, Powell D. Total prosthetic replacement of the TMJ: experience with two systems 1988-1997. Br J Oral Maxillofac Surg. 2000; 38:360. [ Links ]
13. Saeed NR, McLeod NMH, Hensher R. Temporomandibular joint replacement in rheumatoid-induced disease. Br J Oral Maxillofac Surg. 2001; 39:71. [ Links ]
14. Mishima K, Yamada T, Sugahara T. Evaluation of respiratory status and mandibular movement after total temporomandibular joint replacement in patients with rheumatoid arthritis. Int J Oral Maxillofac Surg. 2003; 32:275. [ Links ]
15. Wolford LM, Cottrell DA, Henry C. Sternoclavicular grafts for temporomandibular joint reconstruction. J Oral Maxillofac Surg. 1994; 52:119. [ Links ]
16. Freitas RZ, Mehra P, Wolford LM. Autogenous versus alloplastic TMJ reconstruction in rheumatoid-induced TMJ disease. J Oral Maxillofac Surg. 2002; 58(Suppl 1):43. [ Links ]
17. Mehra P, Wolford LM, Baran S, Cassano DS. Single-stage comprehensive surgical treatment of the rheumatoid arthritis temporomandibular joint patient. J Oral Maxillofac Surg. 2009; 67:1859-72. [ Links ]
18. Wolford LM, Mehra P. Simultaneous temporomandibular joint and mandibular reconstruction in an immunocompromised patient with rheumatoid arthritis: a case report with 7-year follow-up. J Oral Maxillofac Surg. 2001; 59:345. [ Links ]
19. Mercuri LG. Surgical management of TMJ arthritis. In: Laskin D.M., Greene C.S., Hylander W.L., editors. Temporomandibular joint disorders: an evidence-based approach to diagnosis and treatment. Chicago: Quintessence; 2006. 455-68. Chapter 31. [ Links ]
20. Matsuura H, Miyamoto H, Ishimura J, et al. Effect of partial immobilization on reconstruction of ankylosis of the temporomandibular joint with autogenous costochondral graft. Br J Oral Maxillofac Surg. 2001; 39:196. [ Links ]
21. Petty W., editors. Total joint replacement. Philadelphia: Saunders; 1991. [ Links ]
22. Bouyssie JF, Bouyssie S, Sharrock P, et al. Stereolithographic models derived from X-ray computed tomography. Reproduction accuracy. Surg Radiol Anat. 1997; 19:193-9. [ Links ]
23. Pitta MC, Wolford LM. Use of acrylic spheres as spacers in staged temporomandibular joint surgery. J Oral Maxillofac Surg. 2001; 59:704-6. [ Links ]
24. Wolford LM, Karras SC. Autologous fat transplantation around temporomandibular joint total joint prostheses: preliminary treatment outcomes. J Oral Maxillofac Surg. 1997; 55:245. [ Links ]
25. Mercuri LG, Alcheikh Ali F, Woolson R. Outcomes of total alloplastic replacement with peri-articular autogenous fat grafting for management of re-ankylosis of the temporomandibular joint. J Oral Maxillofac Surg. 2008; 66:1794-803. [ Links ]
26. Pearce CS, Cooper C, Speculand B. One stage management of ankylosis of the temporomandibular joint with a custom-made total joint replacement system. Br J Oral Maxillofac Surg. 2009; 47:530-4. [ Links ]
27. Henry CH, Wolford LM. Treatment outcomes for temporomandibular joint reconstruction after proplast-teflon implant failure. J Oral Maxillofac Surg. 1993; 51:352. [ Links ]
28. Mercuri LG. A rationale for alloplastic temporomandibular joint reconstruction in the management of idiopathic/progressive condylar resorption. J Oral Maxillofac Surg. 2007; 65:1600-9. [ Links ]
29. Westermark A, Hedén P, Aagaard E, Cornelius CP. The use of TMJ concepts prostheses to reconstruct patients with major temporomandibular joint and mandibular defects. Int J Oral Maxillofac Surg. 2011; 40:487-96. [ Links ]
30. Mercuri LG, Swift JQ. Considerations for the use of alloplastic temporomandibular joint replacement in the growing patient. J Oral Maxillofac Surg. 2009; 67:1979-90. [ Links ]
31. Wolford LM, Dingworth DJ, Talwar RM, et al. Comparison of 2 temporomandibular joint prosthesis systems. J Oral Maxillofac Surg. 2003; 61:685. [ Links ]
32. Branemark PI, Hansson HA, Adell R. Osseointegrated implants in the treatment of the edentulous jaw: experience from a ten year period. Scan Plast Reconstr Surg. 1977; 11(Suppl 16). [ Links ]
33. Morscher EW. Implant stiffness and its effects on bone and prosthesis fixation. In: Sedel L., Cabanela M.E., editors. Hip surgery: materials and developments. St. Louis: Mosby; 1998. [ Links ]
34. Mercuri LG, Wolford LM, Sanders B, et al. Custom CAD/CAM total temporomandibular joint reconstruction system: preliminary multicenter report. J Oral Maxillofac Surg. 1995; 53:106. [ Links ]
35. Mercuri LG, Wolford LM, Sanders B, et al. Long-term follow-up of the CAD/CAM patient fitted alloplastic total temporomandibular joint reconstruction prosthesis. J Oral Maxillofac Surg. 2002; 60:1440. [ Links ]
36. Mercuri LG, Giobbe-Hurder A. Long-term outcomes after total alloplastic temporomandibular joint reconstruction following exposure to failed materials. J Oral Maxillofac Surg. 2004; 62:1088. [ Links ]
37. Mercuri LG, Edibam NR, Giobbie-Hurder A. 14-year follow-up of a patient fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg. 2007; 65:1140-8. [ Links ]
38. Charnley J. Low friction arthroplasty of the hip: theory and practice. London: Springer-Verlag; 1979. [ Links ]
39. Schmalzried TP, Brown IC. Mechanisms of prosthetic joint failure. In: Gallante J.O., et al, editors. Total hip revision surgery. NY: Raven Press; 1995. [ Links ]
40. Mercuri LG, Anspach W. Principles for the revision of TMJ prostheses. Int J Oral Maxillofac Surg. 2003; 32:353-9. [ Links ]
41. Poggie RA. A review of design, contact stresses, and material wear of metal-on-metal hip prostheses. ASTM STP. 1998; 1346:47-54. [ Links ]
42. Fisher J. Bioengineering reasons for the failure of metal-on-metal hip prostheses: an engineer's perspective. J Bone Joint Surg Br. 2011; 93:1001-4. [ Links ]
43. Hallab NJ: Associate Professor, Orthopaedic Surgery, Rush Medical Center, Chicago, IL. Personal communication. 2011. [ Links ]
44. Salter RB. Continuous passive motion. Baltimore: Williams and Wilkins; 1993. [ Links ]
45. Bradrick JP, Indresano AT. Failure rate of repetitive temporomandibular joint surgical procedures. J Oral Maxillofac Surg. 1992; 50:150. [ Links ]
46. Drangsholt M, LeResche L. Temporomandibular disorder pain. In: Crombie I.K., Croft P.R., Linton S.J., LeResche L., Von Korff M., editors. Epidemiology of pain: a report of the task force on epidemiology of the international association for the study of pain. Seattle: International Association for the Study of Pain; 1999. 203-33. [ Links ]
47. Wolford LM, Rodrigues DB, McPhillips A. Management of the infected temporomandibular joint total joint prosthesis. J Oral Maxillofac Surg. 2010; 68:2810-23. [ Links ]
48. Ghannoum M., O'Toole G.A., editors. Microbial biofilms. Washington, DC: ASM Press; 2004. [ Links ]
49. Donlon RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002; 8:2. [ Links ]
50. Mercuri LG. Microbial biofilms - a potential source of alloplastic device failure. J Oral Maxillofac Surg. 2006; 64:1303-9. [ Links ]
51. Levent T, Vandevelde M, Delobelle JM, et al. Infection risk prevention following total knee arthroplasty. Orthopaed Traumatol Surg Res. 2010; 96:48-55. [ Links ]
52. Shehab D, Abdelhamid H, Collier D. Heterotopic ossification. Nucl Med. 2002; 43:346-53. [ Links ]
53. Ritter MA, Gioe TJ. The effect of indomethacin on para-articular ectopic ossification following total hip arthroplasty. Clin Orthop. 1982; 167:113. [ Links ]
54. Francis MD, Russell RCG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathologic calcifications in vivo. Science. 1969; 165:1264. [ Links ]
55. Reid R, Cooke H. Postoperative ionizing radiation in the management of heterotopic bone formation in the temporomandibular joint. J Oral Maxillofac Surg. 1999; 57:900. [ Links ]
Received 17 January 2012
Accepted 19 February 2012














