Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Cirugía Oral y Maxilofacial
versión On-line ISSN 2173-9161versión impresa ISSN 1130-0558
Rev Esp Cirug Oral y Maxilofac vol.37 no.1 Madrid ene./mar. 2015
https://dx.doi.org/10.1016/j.maxilo.2013.05.007
ORIGINAL ARTICLE
Comparative study of bone regeneration in critical cranial bone defects using bone marrow adult stem cells and calcium phosphate
Estudio comparativo sobre regeneración ósea en defectos óseos craneales con células madre adultas de la médula ósea y fosfato cálcico (BoneSource®)
Marvis Allaisa,b,c, Paul E. Maurettea,b,c, Natália Gomes de Moraisd, Thacianna Barreto da Costad, Simone Fragad, Emanuel Dias de Oliveira e Silvaa, Jose Rodrigues Laureano Filhoa and Celia Maria Barbosa de Castrod
a Department of Oral and Maxillofacial Surgery, Pernambuco Dental School, University of Pernambuco, Recife, Pernambuco, Brazil
b Pathology, Oral Surgery and Oral Medicine Department, Santa María University, Caracas, Venezuela
c Oral Surgery Department, Santa María University, Caracas, Venezuela
d Microbiology Division, Laboratory of Immunopathology Keizo-Asami, Federal University of Pernambuco, Recife, Pernambuco, Brazil
ABSTRACT
Objective: To compare the use of Bone Marrow adult Stem Cells (BMSCs), differentiated in vitro into osteoblasts, associated to calcium phosphate versus autogenous bone graft, in the repair process of critical size bone defects.
Materials and method: On 36 Wistar adult rats, bilateral full-thickness defects on parietal bone were created. The defects were either repaired with calcium phosphate (group I), calcium phosphate + (BMSCs) (group II) or autogenous bone graft (group III), and the opposite side with blood clot (Control Group). In all cases a collagen membrane was used. The animals were sacrificed at 30 and 60 days, and all specimens were collected for further histological and histomorfometric study.
Results: At 30 days, group III (autogenous bone graft) evidences a statistical difference on bone formation when compared to the experimental and control groups (p ≤ 0.05). At 60 days group II (BS + BMSCs) and group III (autogenous bone) showed a similar bone formation and has only a statistical difference when compared to group I (BS) and control group.
Conclusion: The use of calcium phosphate in conjunction with BMSCs resulted in a similar behavior in the process of bone repair in critical size defects, when compared with autogenous bone graft.
Keywords: Bone marrow. Stem cells. Bone regeneration.
RESUMEN
Objetivo: Comparar el uso de células madre adultas de la médula ósea (CMMO), diferenciadas in vitro en osteoblastos, asociadas a fosfato cálcico, frente a injerto de hueso autólogo, en el proceso de reparación de defectos óseos de tamaño crítico.
Material y Método: En 36 ratas adultas Wistar, se crearon defectos bilaterales de todo el grosor en el hueso parietal. Los defectos se repararon con fosfato de calcio (BoneSource®) (grupo I), fosfato de calcio (BoneSource®) + (CMMO) (grupo II) o injerto de hueso autólogo (grupo III), y en el lado contralateral con coágulo de sangre (grupo de control). En todos los casos se utilizó membrana de colágeno. Los animales fueron sacrificados a las 30 y 60 días y se obtuvieron todas las muestras para el estudio histológico y el análisis histomorfométrico.
Resultados: A los 30 días, en el grupo III (injerto de hueso autólogo) se puso de manifiesto una diferencia estadísticamente significativa en la formación de hueso en comparación con el grupo experimental y el de control (p < 0,05). A los 60 días, en el grupo II (BoneSource®) + CMMO) y el grupo III (hueso autólogo) se demostró una formación ósea similar, y sólo se evidenció una diferencia estadísticamente significativa en comparación con el grupo I (BoneSource®) y el grupo de control.
Conclusión: El uso de fosfato de calcio en combinación con CMMO indujo un comportamiento similar en el proceso de reparación ósea en defectos de tamaño crítico, en comparación con injerto de hueso autólogo.
Palabras clave: Médula ósea. Células madre. Regeneración ósea.
Introduction
The reconstruction of severe bone defects represents a challenge for the surgeon. These defects are common in patients who have suffered severe trauma, tumor resections or congenital deformities.1,2 The gold standard treatment is the autogenous bone graft, but the main complications are: morbidity at the site of the second surgery and the limited amount of bone available.2-4 Recently the use of minimal invasive techniques has been reported to show decreased morbidity at the primary operative site. Many materials have been tested to avoid the graft donation surgery; the most common material used in reconstructions is calcium phosphate cement (BoneSource®), a mixture of tetracalcium phosphate (TTCP) and dicalcium phosphate anhydride (DCPA). Investigations have shown that calcium phosphate cement, like other heterogeneous materials, provides two elements required for bone regeneration [osteoconduction and osteoinduction] but does not have osteogenic properties.5-9 Currently, the use of tissue engineering techniques can be used to make bones with these three elements and eliminate the morbidity of the donor graft collection site.3,10,11
The goal of this study was to compare the use of Bone Marrow Adult Stem Cells (BMSCs), differentiated in vitro into osteoblast, used in conjunction of calcium phosphate versus autogenous bone graft, in the process of repairing critical size bone defects.
Materials and methods
Animal model
54 male, adult, Wistar rats (age 120 days; weight, 25 kg) were used for this experiment. The study was performed in agreement, with the regulations and approval of the Institutional Animal Care and Use Committee of the Federal University of Pernambuco-Brazil and according to the standards of the Laboratory Animal Care Assessment and Accreditation Association under number No. 019687/2006-72. The animals were housed for 1 week, for them to become acclimatized to their new housing and diet. Throughout the experiment, the rats were monitored for general appearance, activity, excretion, and weight.
72 full-thickness parietal bone defects of 5 mm in diameter were created bilaterally on 36 Wistar adult rats, 2 in each rat. On the same animal the defects were either repaired with calcium phosphate cement (BoneSource®) - group I, calcium phosphate cement and Bone Marrow adult Stem Cells (BoneSource®) + (BMSCs) - group II, or autogenous bone graft - group III, and the opposite side was treated with a blood clot (Control). Animals were sacrificed at 30 and 60 days (6 out of each group) after the surgery and all specimens were harvested for histological and histomorphometric studies, to assess differences in healing and bone formation between groups.
The collection of bone marrow was made twelve days prior to the surgery, the graft was taken from 18 donor rats' tibias and femurs, at a rate of 3-2, based on reported values of 6 and 9 × 106 cells/cm3. First of all the animals were anesthetized with hydrochloride xylazine and ketamine hydrochloride, then sacrificed with intracardiac potassium chloride. To collect the marrow, the bones were cut at the height of the epiphysis, so that the shafts remained intact and therefore allow for medulla recollection. This was performed directly in a sterile conical Falcon tube of 15 ml with a needle of 18G × 11/2″, using Heparin 1:10 in 0.1 M PBS, pH 7.2. After completing the collection, stem cells were separated on a Ficoll gradient, under centrifugation at 450 turns for 30 min.
The top layer of stem cells above the Ficoll was sucked through an automatic pipette and placed in a sterile conical tube (Falcon, 50 ml). These cells were then washed in PBS, centrifuged under 450 rounds for 10 min. After centrifugation, a precipitate that represents the cells was obtained by discarding the supernatant. The separate cells were resuspended in 1 ml of Dulbecco Modified Eagle Medium (DMEM) with a high rate of glucose (Invitrogen®), supplemented with 10% fetal calf serum (Invitrogen®) and antibiotic/antimycotic (Ampicillin 1 g Invitrogen®). We obtained an aliquot of 10 μl of cell counts, which was suspended in 90 μl of Trypan blue (1:10) and then carried out in a Neubauer chamber, and the result adjusted to 106 cells/ml.
Stem cells were grown in 25 cm2 culture bottles, for 12 days in a humidified incubator with 5% CO2 in sterile conditions. The acquisition of mesenchymal stem cells was through the adhesion of adult stem cells to the bottom of culture flask. The exchange of culture medium was performed every three days and until the tenth day (Fig. 1), when the cultures reached a confluence of 80-90%, they were induced to differentiate into osteoblasts phenotypically by the addition of an induction medium containing 100 nmol/l dexamethasone, 10 nmol/l μ-glycerophosphate and 50 μg/ml of ascorbic acid (Fig. 1).
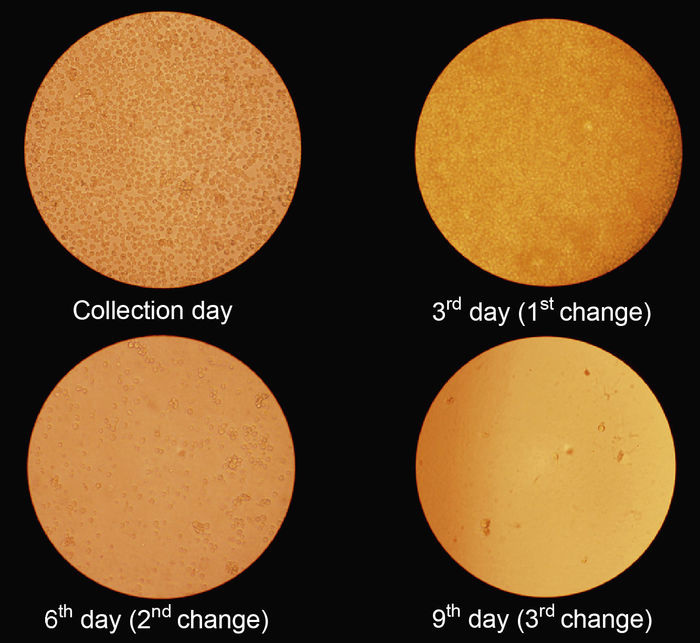
Fig. 1 Stem cells culture. The exchange of culture medium was performed every three days until the tenth day.
At day 12, osteoblasts were removed from the bottle with 5 ml of Trypsin-EDTA (0.05%) washed in PBS and added to a sterile sodium chloride (NaCl) 0.9% solution, a concentration of 106/10 μl NaCl (Fig. 2).

Fig. 2 When the cultures reached a confluence of 80-90%, they
were induced to differentiate into osteoblasts phenotypically.
The calcium phosphate cement (BoneSource®) was divided into sterile plastic tubes (Eppendorf) each one containing 0.25 g of the powder, and was separated for the animals where the material would be tested.
Before the trichotomy was performed, antisepsis of the craniofacial region of the rat was carried out. Local anesthesia was obtained with local infiltration of 0.2 ml lidocaine hydrochloride at 2% with epinephrine concentration of 1:100,000 in order to decrease bleeding in the trans-operative and encouraging greater comfort in the immediate postoperative. Then an incision was made in the skin and periosteum of the cranial region making a full thickness flap, after the skull exposition, two full-thickness, circular bone cavities were made in the central region of each parietal bone, using a trephine drill of 5 mm in diameter. Finally the bone fragment was gently removed with a Lucas curette. The bone perforations were carried out while constantly irrigating with physiological solution of sodium chloride 0.9%, thus avoiding the overheating of bone. Defects were filled at random according to the group (Fig. 3).

Fig. 3 Initial surgery procedure.
Group I
The test cavity was filled with calcium phosphate cement (BoneSource®). The material was prepared with the calcium monophosphate (liquid and powder) and gets mixed up to form a uniform paste.
Group II
The test cavity was filled with powder of calcium phosphate cement (BoneSource®) associated with Bone Marrow adults stem cells (BMSCs), differentiated in vitro in osteoblast. (It was mixed up the cement powder of BoneSource®) and 10 microliters of suspension containing one million (1 × 106) of cells approximately).
Group III
In this group the bone fragments removed with the trephine drill were placed in the cavity (Fig. 4).

Fig. 4 Filled defects randomly according to the Group.
In all cases a collagen membrane was used to avoid migration of materials and any interference of the periosteum on the regeneration process. Finally the wound was sutured (Fig. 5).

Fig. 5 Final surgery procedure.
Histological analysis
Three pathologists accomplished the histological analysis. All the researchers were university professors in tissue and bone lesions analysis. The three assessors had limited knowledge about the methodology of this experiment, not knowing which was the group or cavity to be evaluated.
For the evaluation of the samples the plates were delivered in boxes labeled with HE staining and TM. Additionally each judge was handed a folder containing a standardized form, categorized as *1 = present and *2 = absent. The histological parameters used were: osteoid, immature bone, mature bone, newly formed vessels, granulation tissue, collagen fibers, fibroblasts, osteocytes, osteoclasts, osteoblasts, and inflammatory infiltrate implanted material. The item inflammatory infiltrate was categorized as *0 = absent, *1 = acute, *2 = chronic.
Histomorphometric analysis
The evaluation and quantification of newly formed bone tissue was performed at the Faculty of Dentistry, Bahia Foundation for the Advancement of Science, in Salvador Bahia, through the use of the program Motic Images Advanced 3.0 (Micro-Optic Industrial Group Co. Ltd.) compatible with Windows XP operating system. Initially, the sections stained with HE were examined with a light microscope with an increase of 4 and 10× to ensure complete visualization of the original defect, then being captured by digital camera attached to the microscopic system Motic Images Advanced 3.0 and displayed on the computer monitor and were captured in slide images at 4, 10 and 40×. Progressive numbering sorted by operators who participated directly in the research but without knowing to which group it belonged performed the capture of images on the blades.
Results
This study showed that the defect of 5 mm in the calvarial bone formation was limited to the margins of the defect without revealing spontaneous bone regeneration after 4 or 8 weeks of surgery (Fig. 5).
The time working with both, the sodium monophosphate and the solution containing osteoprogenitor cells, was enough for proper handling of the material, allowing an execution without major technical difficulties. Surgical time did not increase due to the manipulation of the suspension of osteoprogenitor cells.
The bone formation in experimental groups was always higher than control groups, which were filled with blood clot. In periods of 30 and 60 days there was a significant difference between all the materials and the control group (p ≤ 0.001). In control group the media in bone formation were similar in all groups and periods of sacrifice, showing no bone formation (Table 1).
Table 1 Histomorphometric evaluation of the difference between test and control groups. 
Comments: If all the letters in parentheses are different statistical significance differences exist
between materials. a - SD = Standard Deviation.
b - Statistical test F (ANOVA) with Tukey comparisons in case of Statistical significance.
c - Through test t-Student. * - Statistical significance 5.0%.
When new bone formation was compared between the three experimental groups, it was observed that the period of 30 days in group III (autogenous bone) had the highest average of new bone formation when compared with the other two groups (19805.58 ± 1507.94), with a significant difference at p ≤ 0.001. Also in this period of sacrifice, group II (cement + CT) had a higher average than group I (cement) but no significant difference between both (16293.02 ± 1140.50 > 14981.32 ± 1837.72). When comparing the averages in the second period of sacrifice (60 days) both group II and group III had similar average with a higher bone formation when compared to group I, with a significant difference at p ≤ 0.004. This period of sacrifice in group II had a higher mean than group III, but no significant difference between both materials (Table 2 and Fig. 6).
Table 2 Histomorphometric evaluation of tested material and time of sacrifice. 
Comments: If all the letters in parentheses are different Statistical Significance differences exist
between materials. * - Statistical significance 5.0%.
a - SD = Standard Deviation.
b - Statistical test F (ANOVA) with Tukey comparisons in case of statistical significance.
c - Through test t-Student.

Fig. 6 Images of histomorphometric analysis.
Discussion
The calcium phosphate cement (BoneSource®) has been widely used to repair defects in the craniofacial region, its use began in 1997 when the Food and Drug Administration (FDA) approved its application, the first such material available for use, being tested in clinical presentations in multiple studies. It is an osteoconductive material in its pure form that is converted into hydroxyapatite cement, with the same properties found in natural bone, but it does not have osteoprogenitor cells.12-14
This material was chosen for this study due to its many properties; its presentation (liquid and powder) makes it is easy to handle, it has excellent biocompatibility in the literature and market availability. In most studies of stem cells associated with the filling materials the ones commonly chosen were coral scaffolds or hydroxyapatite, mainly because the goal was to assess bone formation within the pores of the framework. In this study we used calcium phosphate cement (BoneSource®) due to which our goals were met by the clinical application of this technique, in cases were filling would required it would be much more feasible and economical than the construction of scaffolds by default.
Contrary to what may be led to believe by this clinical trial, the surgery time has not increased due to the manipulation of osteoprogenitor cell suspension, which leads us to believe that its clinical application shall not increase the costs due to the need for a prolonged surgical procedure in reconstructing bone.
With the histological results of three evaluators, a Kappa test was performed to assess the level of agreement in the answers between pairs of examiners. Based on the results the examiners were calibrated because only two items for the evaluation has a different result among them.
About the calcium phosphate cement in this study, we observed that, between weeks 4 and 8, the presence of multinucleated giant cells was a constant finding and location of these cells was always close to the particles of the material. The decaling process for preparing the specimen for histological evaluation left a space corresponding to the particle of calcium phosphate cement and it was possible to identify the inflammatory infiltrate in the vicinity of these spaces. These findings are consistent with reports from Indovina Jr and Block (2002)15 who observed an infiltration of mononuclear cells and multinucleated giant cells around areas related to the particles of the implanted material, even after a period of 8 and 12 weeks after surgery.
The bone formation in experimental groups of this study was always higher in the test cavities receiving a filling material than in control cavities that were filled with blood clot from the animal itself. In periods of 30 and 60 days there was no significant difference between all the materials and the controls (p ≤ 0.001). The bone formation average in the control groups remained similar in all groups and periods of sacrifice, showing that nobone formation in critical defects, in agreement with literature reports.6,16 Groups I and II showed a significant difference in bone formation, when we compared the main differences in histomorphometry between groups and the two sacrifice periods, as in group III this value was similar in both periods showing the same behavior during the treatment.
When the new bone formation was compared between the 3 groups, we observed that the period of 30 days in group III (autograft) had the highest average of new bone formation when compared with the other two groups with a significant difference. Also in this period of sacrifice, group II (cement + CT) had a higher average than group I (cement) but there was no significant difference between them. When comparing the averages in the second period of sacrifice (60 days) it was found that both group II and group III had similar medium with a higher bone formation when compared to group I, with a significant difference. This period of sacrifice group II had a higher average than group III, but no significant difference between both materials. These results can be compared with literature reports, where there was bone formation in regions treated with stem cell culture.3,17-19
Several works show the formation of bone tissue using culture of mesenchymal cells into scaffolds implanted in specific or ectopic areas; a study published by Abukawa et al. in 200317 shows by the making a framework shaped condyle filled with osteoprogenitor cells, and implanted on the backs of mice, there was bone formation, but only on the surface and without vascular formation. They concluded that more studies should be performed to improve the penetration of cells in the scaffold, and promote vascular formation. The following year the same authors published a study where the protocol for stem cell culture, the framework and time to deploy the material was changed, trying to reach the bone formation in an even way, and with central vascularization. They reached the goals and concluded that mesenchymal cells derived from bone marrow can be manipulated and when placed in an environment or framework can form a structure similar to the actual bone.3
The results presented here may be related to these works through a mixture of osteoprogenitor cells derived from bone marrow mesenchymal cells associated with a vehicle such as cement of calcium phosphate. We found bone formation in defects filled with this material at 60 days. The material presented in average a greater bone formation than autologous bone graft, but no significant difference between the two, leading us to believe that this type of treatment may be the key to success in tissue engineering regarding bone reconstruction, apparently it also had the same characteristics as autograft.
Mesenchymal stem cells (MSCs) derived from bone marrow have been used in the repair of different tissues such as cartilage or bone due to its ability to transform into specific cells after the culture in different medium, the use of β-glicerolfosfato and dexamethasone can improve the osteogenic phenotype of these cells,10,19,20 but the need for removal of large quantities of bone marrow for a few mesenchymal cells is a major problem in the cells obtained from that source.
Studies published recently show that for every 100,000 cells obtained from bone marrow only one can be considered a mesenchymal cell, which indicates that only a limited proportion of cells can be differentiated into osteogenic lineage.21,22 In this work we observed this complication and later in the pilot study, we had intended to obtain cells from one donor mouse to a recipient mouse, but the amount of cells present was so low it was necessary to increase the number of donor rats to 3, which generates a greater number of dead animals, a greater number of materials used and a much higher waste of time and money.
Solutions for this problem: several studies have been recently published using cells derived from adipose tissue.23,24 In a report by Cui et al. in 2007,25 they repaired critical defects in ice dogs by coral frameworks associated with the culture of stem cells derived from body fat and concluded that there was enough bone formation to be used in this cell type and underlined it as an alternative source of abundant stem cells.
The bone in the maxillofacial region has a key role in maintaining both the facial function and appearance. Facial anatomy should be restored correctly when it becomes compromised by any pathology. The future in bone reconstruction will apply predefined scaffolds loaded with mature osteoprogenitor cells and/or embryo cells cultured in vitro to reconstruct maxillofacial defects and cover them entirely.
Conclusion
Bone marrow stem cells used in conjunction with a calcium phosphate cement were similar to the gold standard autogenous bone grafts in bone regeneration of critical size defects. It provided the three elements required for bone regeneration: osteoconduction, osteoinduction, and osteogenic cells.
We observed that the 5 mm critical size defects treated with no material, i.e. blood clot, showed no bone regeneration as the use of a collagen membrane avoided migration of material into these control sites at 4 and 8 weeks post-surgery.
Surgical time was not altered by the use of stem cells in conjunction with the calcium phosphate cement so its clinical application will not require longer time periods compared to conventional procedures.
Ethical approval
The study was performed in agreement, with the regulations and approval of the Institutional Animal Care and Use Committee of the Federal University of Pernambuco-Brazil and according to the standards of the Laboratory Animal Care Assessment and Accreditation Association under number No. 019687/2006-72. The animals were housed for 1 week, for them to become acclimatized to their new housing and diet. Throughout the experiment, the rats were monitored for general appearance, activity, excretion, and weight.
Ethical disclosure
Protection of people and animals. The authors declare that procedures conformed to the ethical standards of the responsible committee on human experimentation and in accordance with the World Medical Association Declaration of Helsinki.
Confidentiality of data. The authors declare that they have followed the protocols of their workplace on the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consent. The authors have obtained the informed consent of patients and/or subjects referred to in the article. This document is in the possession of the author of correspondence.
Funding.- None.
Conflict of interest
None declared.
Acknowledgment
None.
References
1. Clokie C.M., Moghadam H., Jackson M.T., Sandor G.K. Closure of critical sized defects with allogenic and alloplastic bone substitutes. J Craniofac Surg 2002;13:111-21. [ Links ]
2. Mazzonetto R., Allais M., Maurette P.E., Moreira R.W. A retrospective study of the potential complications during alveolar distraction osteogenesis in 55 patients. Int J Oral Maxillofac Surg 2007;36:6-10. [ Links ]
3. Abukawa H., Shin M., Williams W.B., Vacanti J.P., Kaban L.B., Troulis M.J. Reconstruction of mandibular defects with autologous tissue-engineered bone. J Oral Maxillofac Surg 2004;62:601-6. [ Links ]
4. Mazzonetto R., Allais M. Radiographic evaluation of alveolar distraction osteogenesis: analysis of 60 cases. J Oral Maxillofac Surg 2005;63:1708-11. [ Links ]
5. Ambard A.J., Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont 2006;15:321-8. [ Links ]
6. Gómez E., Martín M., Arias J., Carceller F. Clinical applications of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children: our experience at Hospital La Paz since 2001. J Oral Maxillofac Surg 2005;63:8-14. [ Links ]
7. Kirschner R.E., Karmacharya J., Ong G., Gordon A.D., Hunenko O., Losee J.E. Repair of the immature craniofacial skeleton with a calcium phosphate cement: quantitative assessment of craniofacial growth. Ann Plast Surg 2002;49:33-8. [ Links ]
8. Rabie A.B., Chay S.H., Wong A.M. Healing of autogenous intramembranous bone in the presence and absence of homologous demineralized intramembranous bone. Am J Orthod Dentofacial Orthop 2000;117:288-9. [ Links ]
9. Schmitz J.P., Hollinger J.O., Milam S.B. Reconstruction of bone using calcium phosphate bone cements: a critical review. J Oral Maxillofac Surg 1999;57:1122-6. [ Links ]
10. Bianco P., Riminucci M., Gronthos S., Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001;19:180-92. [ Links ]
11. Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7. [ Links ]
12. Crawford K., Berrey B.H., Pierce W.A., Welch R.D. In vitro strength comparison of hydroxyapatite cement and polymethylmethacrylate in subchondral defects in caprine femora. J Orthop Res 1998;16:715-9. [ Links ]
13. Dickson K.F., Friedman J., Buchholz J.G., Flandry F.D. The use of Bone Source hydroxyapatite cement for traumatic metaphyseal bone void filling. J Trauma 2002;53:1103-8. [ Links ]
14. Miyamoto Y., Ishikawa K., Fukao H., Sawada M., Nagayama M., Kon M. In vivo setting behaviour of fast-setting calcium phosphate cement. Biomaterials 1995;16:855-60. [ Links ]
15. Indovina A., Block M.S. Comparison of 3 bone substitutes in canine extraction sites. J Oral Maxillofac Surg 2002;53-8. [ Links ]
16. Aybar A., Territoriale E., Missana L. An experimental model in calvaria to evaluate bone therapies. Acta Odontol Latinoam 2005;18:63-7. [ Links ]
17. Abukawa H., Terai H., Hannouche D., Vacanti J.P., Kaban L.B., Troulis M.J. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg 2003;61:94-100. [ Links ]
18. Caplan A.I. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 2005;11:1198-211. [ Links ]
19. Chen F., Mao T., Tao K., Chen S., Ding G., Gu X. Bone graft in the shape of human mandibular condyle reconstruction via seeding marrow-derived osteoblasts into porous coral in a nude mice model. J Oral Maxillofac Surg 2002;60:1155-9. [ Links ]
20. Martin I., Padera R.F., Vunjak-Novakovic G., Freed L.E. In vitro differentiation of chick embryo bone marrow stromal cells into cartilaginous and bone-like tissues. J Orthop Res 1998;16:181. [ Links ]
21. Kim S., Kim S.S., Lee S.H., Eun S., Gwak S.J., Song J.H. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly (D,L-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials 2008;29:1043-53. [ Links ]
22. Verfaillie C.M. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol 2002;12:502-8. [ Links ]
23. Dudas J.R., Marra K.G., Cooper G.M., Penascino V.M., Mooney M.P., Jiang S. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg 2006;56:543-8. [ Links ]
24. Hicok K.C., Du T.V., Zhou Y.S., Halvorsen Y.D., Hitt D.C., Cooper L.F. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng 2004;10:371-80. [ Links ]
25. Cui L., Liu B., Liu G., Zhang W., Cen L., Sun J. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 2007;28:5477-86. [ Links ]
![]() Correspondence:
Correspondence:
E-mail addresses: marvisallais@gmail.com, m.allais@me.com (M. Allais).
Received 3 May, 2013
Accepted 23 May, 2013














