Introduction
Oral cancer is one of the most frequent cancers of the head and neck region, and represents between 2 and 4% of all malignant tumors. Approximately 90% of oral malignancies are squamous cell carcinoma (SCC). Oral SCC (OSCC) is more frequent in males and in patients older than 60 years, and its etiology is multifactorial. It is frequently associated with habits of tobacco or alcohol consumption or bad oral hygiene, but other factors, such as infections (viral o bacterial) or immunosuppression can also be implicated.1,2 This type of cancer originates from the stratified squamous epithelium of the oral cavity, and irritative or traumatic factors seem to play a role in its development.2
OSCC has a local recurrence rate of about 20%.3 Second primary tumors of the mouth are also not infrequent, and there is currently an increase in the female population and in patients younger that 40, even in non-smokers. When a suspicious lesion appears in a patient with a past history of oral tumors, a differential diagnosis between local recurrence and second primary should take place. To exclude the possibility of local recurrence the following must be considered: a second primary tumor has to be at least 2 cm away from the primary tumor, and 3 years must have passed from the diagnosis of the primary tumor.4
Osteointegrated implants are a safe and efficient technique for dental rehabilitation, and also for oral rehabilitation after surgical resection of oral tumors.4,5 Dental implants (DI) are used more and more by implantologists and maxillofacial surgeons due to their success in these past decades, but they are not free of complications. One of the most common complications of DI is peri-implantitis (PI), which is in an inflammatory process that affects the soft tissues and the bone surrounding the implants. Its cause is multifactorial, and usually presents itself as a swelling of the gum (erythema, hyperplasia or ulcer), with formation of peri-implant pockets due to surrounding bone loss.1,4-8 Clinically, OSCC could be confused with PI (gingival inflammation, tendency to bleed and bone loss), so a correct differential diagnosis is necessary and one must recur to a histologic diagnosis in many cases.7,8 A biopsy is recommended mainly in highly-suspicious cases, for example in a long-lasting swelling of an area surrounding a dental implant that has not healed after conventional treatment,9 or if its appearance is sudden and severe.4
The objective of this article is to revise a cohort of patients with a past history of cancer who were rehabilitated with dental implants (DI). Additionally, we detected 2 cases of carcinoma that appeared in patients that did not have a past history of cancer. A systematic review of published articles and case reports was also done to find an association between dental implants and OSCC.
Materials and methods
A retrospective study was done of cases of implant-related malignancies diagnosed in our center between 2008 and 2017. Data of age, gender, risk factors, clinical presentation, tumor location, previous treatments, follow-up and time-lapse between implant placement and tumor diagnosis was also retrieved. The patients were free of tumor, or had no apparent lesion at the time of the implant placement.
A systematic review of articles and case reports published in medical literature was conducted (up to May 2017) using Medline (PubMed), Cochrane Database and Google Scholar, using the search terms “cancer”, “squamous cell carcinoma”, “oral cancer” and “dental implants”, “dental rehabilitation” “dental implant complications”. The Boolean operator “AND” was used to find an association between dental implants and OSCC. Searches were also carried out of the articles listed in the Bibliography of the articles reviewed to identify relevant studies that might have been omitted. The search was restricted to articles published in English or Spanish, or abstracts in English.
Results
Between 2008 and 2017, a total of 6 cases of implant-related SCC reported. There were only four cases (66.6%) in which the patient had a previous history of OSCC, all of them treated and in follow-up in our center. Two patients developed malignancies surrounding implants with no past history of cancer or of pre-malignant lesions; one of them was a smoker and the other patient did not even have any risk factors.
Four of the patients were women and two of them were men, and the mean age was 66.8 years old at the time of diagnosis (SD ± 9.9, range 51-79). Three of the patients had typical risk factors (tobacco or alcohol consumption), and two of them had previously received radiotherapy. The average time lapse between the placement of the implants and the diagnosis of the neoplastic lesion was 57.9 months (SD ± 34.2, range 24-96 months). In five of the patients, the tumor was located in the mandible, and in one of them it was of the maxilla.
The patient's characteristics are summarized in Table 1.
Table 1 Summary of 6 cases of SCC surrounding implants diagnosed in our center.
| Sex/age | Time-lapse implant placement-diagnosis | Risk factors | Previous history of OSCC | Premalignant lesion | Location | Previous RT | Treatment | Present situation | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F, 79 | 2 yrs | No | No | No | Mandible | No | Surgery+RT+CT | Died 4 months after initial diagnosis |
| 2 | M, 71 | 8 yrs | Ex-smoker/alcohol consumption | Yes | No | Mandible | Yes | Surgery+RT | In follow-up 6 yrs later |
| 3 | F, 62 | 2 yrs | Ex-smoker | Yes | Verrucous dysplasia | Maxilla | No | Surgery | Tumoral recurrence. In follow-up |
| 4 | F, 74 | 6 yrs | No | Yes | No | Mandible | No | Surgery | Died of metastasis |
| 5 | F, 64 | 8 yrs | No | Yes | No | Mandible | Yes | Surgery | In follow-up 1 yr after surgery |
| 6 | M, 51 | 3 yrs | Smoker | No | No | Mandible | No | Surgery | Died of hepatic encephalopathy and septic shock |
Summary of our series of 6 cases.
M: male; F: female; OSCC: oral squamous cell carcinoma; RT: radiotherapy; CT: chemotherapy.
Case 1
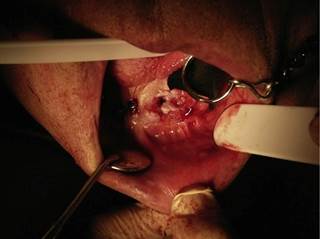
Female patient with no risk factors or previous history of cancer was diagnosed of OSCC at the age of 79 years old after the appearance of a lesion in the posterior sector of the jaw, accompanied by a bone loss of 3 cm surrounding one of two dental implants placed 3 years previous (Fig. 1). The tumor was resected, and the patient received adjuvant radio and chemotherapy due to the diagnosis of basaloid-SCC with presence of multiple ipsilateral lymph nodes. The progress of the disease was quick, and the patient died four months after the initial diagnosis.
Case 2
Male patient, an ex-smoker and ex-alcoholic, had a past history of OSCC located in the floor of the mouth on the right side. The patient presented a tumoral relapse four years later and was treated with a segmental resection of the jaw, reconstructed with a vascularized osteocutaneous fibula flap, and rehabilitated with implants 2 years later. 14 years after the primary tumor, the patient was diagnosed with a second primary tumor of the posterior sector of the jaw on the left side, receiving conservative surgery and radiation therapy. Following the surgery, the patient presented various episodes of mucositis surrounding the anterior implants (located in the skin paddle) that were treated conservatively. Eight years after placement of the DI and a year after the diagnosis of the second primary tumor, a lesion appeared between the mucosa and the island of skin of the fibula flap (Fig. 2), and was diagnosed with a third primary tumor (poorly differentiated SCC). Osseous resective surgery was carried out, and reconstructed with another fibula flap. The patient received adjuvant radiotherapy, and currently is in follow-up 6 years after treatment.
Case 3
Female patient, ex-smoker, had a past history of SCC of the lower jaw. Two years after the placement of DI in the upper maxilla, the patient was diagnosed with a verrucous-type SCC (Fig. 3), and reconstructed with a buccal fat pad. Afterwards, the patient presented tumoral recurrence and second primary tumors of the mouth, which all required surgical treatment.
Case 4
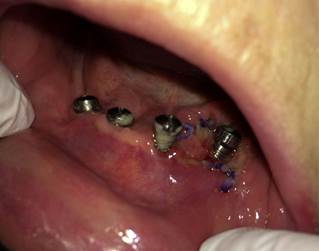
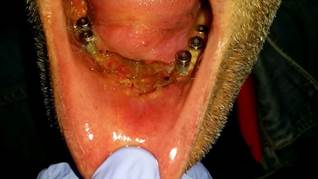
Female patient, with a past history of leukoplakia, moderate-to-intense dysplasias and SCC of the jaw, was treated with surgery and reconstructed using a forearm flap. The patient was rehabilitated with DI in the upper maxilla and the jaw. 6 years later, a leukoplasic-granulomatous lesion appeared in the lower jaw (Fig. 4) adjacent to one of the implants (Fig. 5), which turned out to be a moderately differentiated SCC. A resection of the lesion and a marginal mandibulectomy was done, reconstructed with a buccinator muscle-based myomucosal flap. Years later, the patient has presented various second primary tumors, which have required surgical treatment and chemo and/or radiotherapy. Thirteen years after the primary tumor, the patient was diagnosed with progression of the disease, with local recurrence, extension to lymph nodes and distant metastasis resulting in the death of the patient.
Case 5
Female patient, with a past history of carcinoma of the tongue, treated with a partial glossectomy, ipsilateral neck dissection and adjuvant radiotherapy, was rehabilitated with implants in the jaw. Eight years after their placement, the patient presented cellulitis of the mental region with no bone lesions, but with loss of dental implant integration. Removal of the implant and curettage was done, obtaining the diagnosis of a well-differentiated OSCC. A segmented resection of the lesion was done, and reconstructed with a fibula free flap. The patient currently is in follow-up.
Case 6
Male patient, with no previous history of cancer and tobacco as a sole risk factor, presented a lesion surrounding implants of the anterior sector of the mandible, with infiltration of the floor of the oral cavity and of the lip (Fig. 6). Biopsy was positive for OSCC. The patient was operated on, with resection of the mandible (from angle to angle), the ventral face of the tongue, and the skin of the chin (Fig. 7); the patient was reconstructed with a fibula free flap and a pectoralis major flap. The patient died of grade IV hepatic encephalopathy and septic shock during hospitalization after surgery.
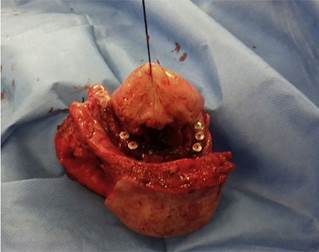
Fig. 7 Sample of the resection of case 6, which included the jaw (from angle to angle), the ventral face of the tongue and the skin of the chin.
A literature search was carried out to find an association between dental implants and OSCC. This search retrieved 54 cases (in 25 articles) published between 1996 and 2017 (Table 2).1,4-27 Of these, 23 patients (42.6%) had a previous history of OSCC (1 of them verrucous-type), and the remaining 31 (57.4%) did not.
Table 2 Systematic review of articles and case reports where SCC is associated with dental implants.
| Authors (year) | Journal name | Number of cases | Risk factors (tobacco/alcohol) | Past history of OSCC | Premalignant lesion | Location | |
|---|---|---|---|---|---|---|---|
| 1 | Clapp et al., 1996 | Arch Otolaryngol Head Neck Surg | 3 | Yes (2); no (1) | No (3) | Yes (1 dysplasia); no (2) | Mandible |
| 2 | Moxley et al., 1997 | J Oral Maxillofac Surg | 1 | Yes | Yes | No | Mandible |
| 3 | Block et Scheufler, 2001 | J Oral Maxillofac Surg | 1 | Yes | Yes (verrucous carcinoma) | Yes (leukoplakia) | Mandible |
| 4 | Shaw et al., 2004 | Int J Oral Maxillofac Surg | 2 | No (2) | Yes (2) | Yes (leukoplakia); no (1) | Mandible |
| 5 | Czerninski et al., 2006 | Quintessence Int | 2 | Yes (2) | Yes (1); no (1) | Yes (1 lichen); no (1) | Mandible |
| 6 | Abu El-Naaj et al., 2007 | Rev Stomatol Chir Maxillofac | 2 | Yes (1); no (1) | No (2) | Yes (lichen); no (1) | Mandible |
| 7 | Schache et al., 2008 | Br J Oral Maxillofacial Surg | 1 | No | No | No | Mandible |
| 8 | Chimenos-Küstner et al., 2008 | Rev Port Estomatol Cir Maxilofac | 1 | Yes | No | No | Mandible |
| 9 | Eguia del Valle et al., 2008 | Med Oral Patol Oral Cir bucal | 1 | No | No | No | Mandible |
| 10 | Gallego et al., 2008 | J Am Dent Assoc | 1 | No | Yes | Yes (lichen) | Mandible |
| 11 | Kwok et al., 2008 | Br Dent J | 3 | Yes (3) | Yes (1) No (2) | No (3) | Mandible |
| 12 | Gallego et al., 2009 | Demt Traumatol | 1 | Yes | No | Yes (lichen) | Mandible |
| 13 | Gulati et al., 2009 | Ann R Coll Surg Engl | 1 | Yes | Yes | Yes (leukoplakia) | Mandible |
| 14 | De Ceulaer et al., 2010 | J Oral Maxillofac Surg | 3 | ? (3) | Yes (3) | No (3) | Mandible |
| 15 | Meijer et al., 2010 | J Oral Maxillofac Surg | 1 | ? | Yes | No | Mandible |
| 16 | Moshref et al., 2011 | J Clin Exp Dent | 1 | No | No | No | Mandible |
| 17 | Bhatavadekar 2012 | J Oral Implantol | 1 | No | No | No | Maxilla |
| 18 | Jané-Salas et al., 2012 | Med Oral Patol Oral Cir Bucal | 2 | Yes (1); no (1) | No (2) | No (2) | Tongue |
| 19 | Marini et al., 2013 | J Oral Maxillofac Surg | 1 | No | No | Yes (lichen) | Mandible |
| 20 | Moergel et al., 2014 | Clin Oral Invest | 15 | Yes (8); no (4); ? (3) | Yes (9); no (6) | Yes (10 leukoplakia, 2 erythroplakia, 2 lichen); no (1) | Mandible |
| 21 | Nariai et al., 2015 | J Oral Maxillofac Surg | 1 | Yes | Yes | No | Mandible |
| 22 | Bhandari et al., 2016 | J Prosthet Dent | 1 | No | No | No | Maxilla |
| 23 | Raiser et al., 2016 | J Oral Maxillofac Surg | 2 | No (2) | No (2) | Yes (2 lichen) | Mandible |
| 24 | Norton 2017 | Br Dent J | 1 | No | No | No | Mandible |
| 25 | Kaplan et al., 2017 | Oral Surg Oral Med Oral Pathol Oral Radiol | 5 | ? (5) | Yes (1); no (4) | Yes (3: 2 leukoplakia, 1 lichen); no (2) | Mandible (3) Maxilla (2) |
Summary of cases published in medical literature of OSCC surrounding implants.
OSCC: oral squamous cell carcinoma; ? - unknown or not recorded.
42.6% of the patients had the risk factor of tobacco or alcohol consumption, 35.2% of them did not, and in 22.2% of them this information was unknown. 51.9% of the patients (n = 28) had some type of pre-malignant lesion: 10 cases of lichen planus, 15 of leukoplakia, 2 of erythroplakia and 1 of mild dysplasia; the remaining 26 patients (48.1%) did not have any of these lesions (Fig. 8). In reference to the location, 48 of the tumors were of the jaw, 5 of the maxilla and 2 of the tongue. Of the 18 patients that had no past oncological history or pre-malignant lesion (33.3%), 8 of them (14.8% of the global number) did not have any risk factors either.
Discussion
Carcinoma surrounding implants seems to be a possibility that needs to be diagnosed as soon as possible as its clinical similarity to processes of PI can result in a delay in diagnosis. According to articles published, SCC surrounding implants can present itself as a mass, as a simple inflammation or as an ulcerated lesion. The male:female ratio is 1:1.5, and there is a preference for the mandibular mucosa.8
Bhatavadekar et al.6 calculated a theoretical SIR (standardized incidence ratio) of SCC after dental implant placement of 0.00017 per 1,000,000 people per year. In comparison, the SIR of cancer after irradiation is 20 per 1,000,000 people per year. Considering the great number of implants that are placed, and the low number of associated SCC reported (49 published, although this number could be higher), the risk can be considered to be very low. To recommend a biopsy of every PI would be considered out of proportion.
Moergel et al.26 carried out a retrospective analysis of the patients who received dental implants over a period of 16 years to find an association between implants and the appearance of OSCC. Of the 2893 patients that received implants, 15 were diagnosed with tumors that emerged directly next to an implant (incidence rate of 0.051%). But in the sub-cohort of patients rehabilitated with implants after tumoral resection (n = 297), 9 of them developed SCC surrounding an implant, with a calculated incidence rate of 3%. This 3% is not very alarming, as the overall risk of tumoral recurrence of OSCC has been reported of up to one-third of the patients diagnosed.28,29
Different authors have different hypotheses of which factors intervene in the malignant degeneration of the tissue surrounding a dental implant. Some authors argue that a possible carcinogenic effect of the metal, based on studies of hip prostheses that describe an increase in the incidence rate of hematological tumors and lymphomas.6 However, this carcinogenic role of the implants per se is unknown, or has not been demonstrated.4,5 Even though titanium is one of the most inert metallic ions with a very low corrosive rate (0.003 μA/cm2),2 situations of inflammation such as with periimplantitis could upset the protective layer of the implant (titanium dioxide) favoring a possible corrosion.6 This swelling accompanied by an increase in acidity, could release of compounds such as eicosanoids, collagenases and prostaglandins E2 that produce bone resorption.
Other authors defend the idea that implants are a gateway into the bone. Schache et al.16) published a case of direct association between an osteointegrated implant and a primary tumor, in a patient with no previous history of cancer or other risk factors. The tumor had originated in the bone crest, and followed the direction of the implant, and centered around it. We present a similar case in our series (case 5). With this theory, the placement of implants can contribute to the development of a SCC that has originated in the epithelium, and extends toward the cancellous bone using the implant as a gateway for the malignant cells to the bone.1 This theory is difficult to defend as a space exists surrounding natural teeth, and there is no reason to believe that the space surrounding an implant is biologically different and would favor a tumoral invasion.6 Another option is that an un-diagnosed malignant or pre-malignant lesion in the area previous to the placement of the implant was present, and that the implant simply carried tumoral cells into the bone. Ultimately, the influence of dental implants in jaw invasion is unknown.4
Radiotherapy as the source of malignant degeneration is another theory. Fukumoto et al.30) studied radio-induced tumors, and reached the conclusion that the following conditions had to be met: be histologically different from the primary tumor, have a latency period of at least 5 years but less that 10 years, and be within the radiation field. De Celular et al.23 published three cases of SCC recurrence surrounding dental implants, in which 2 of them had received radiotherapy after the primary resection. These authors propose a theory of a negative influence of the implant on the radiation field and the dose of radiation, possibly due to the shadow-effect that the implant may have. Of our own series, two patients with a past history of oral tumors had also received radiotherapy after the primary resection. One of them (case 2) had a history of oral SCC, received post-surgical radiation after a second primary tumor, and was diagnosed with a third primary tumor a year later. The other case (case 5) had a second primary tumor in the anterior region of the jaw, after receiving adjuvant radiotherapy after glossectomy due to SCC of the tongue. In both cases, the location of the carcinoma surrounding the implant was within the boundaries of the radiation field that the patient had received.
Chronic swelling appears to be one of the closely associated risk factors to develop OSCC. Virshow in 1863 was the first to describe a possible association between chronic inflammation and cancer. The persistence of this swelling could induce cellular proliferation and cell survival extension by activating oncogenes and inactivating tumor suppressor genes.2 There are various studies that defend the relationship between chronic inflammation and the development of a malignant tumor (such as Crohn's disease and colorectal cancer, or Barrett's esophagus and esophageal adenocarcinoma).8 One theory is that constant inflammation of the gingival tissue surrounding dental implants could induce carcinogenesis caused by cytokine mediators (such as prostaglandins, interleukin-1, interleukin-6 and tumoral necrosis factor).1 Implants could be the cause of the initial swelling, especially in sensitized or at-risk tissue (such as in an alcoholic or a smoker), or be implicated in the increase of inflammation.4 Other theories establish the cause as a disturbance in the microenvironment of the cell, although in contradiction to this theory is the wide range of timeframes between the placement of the DI and the diagnosis of the tumor.8
None of these theories have been proven, but what seems to be clear is that implants can cause a state of chronic inflammation in a patient at risk or with a previous history of cancer, and that this may be associated with the appearance or recurrence of OSCC. Patients with previous intraoral SCC that are operated on and later receive dental rehabilitation require a close follow-up, with a regular physical examination of the peri-implant area, and a biopsy of any suspicious lesion or lesion that does not cure within a reasonable timeframe, especially in patients in which curettage is used as part of the treatment of PI.
A biopsy can also be useful for the diagnosis of other pathologies surrounding implants. In the systematic review of articles, other malignant lesions concurrent with DI were also found: 5 cases of bone metastasis to the jaw (3 cases of lung cancer and 3 of breast cancer). The literature review also retrieved one case of osteosarcoma and another case of type-B lymphoma (neither patient had any risk factor or past oncological history). Another case of basal cell carcinoma was also identified in a patient that had the same lesion in other intraoral locations.
The presence of intraoral lesions should also be taken in consideration when contemplating dental rehabilitation with implants. Lichen planus is a pre-neoplastic condition with a rate of malignant transformation risk of 1%5 (between 0 and 12.5%31). That dental implants favor this malignant transformation of premalignant lesions is unknown and should be further studied in the future.
Conclusion
Although there are no studies that prove a direct relationship between the presence of dental implants and the risk of SCC, it appears that chronic inflammation of the tissue surrounding the implant could be an important factor. The incidence rate of SCC surrounding DI seems to be high in patients with previous oral tumors, and very low outside of this group. In conclusion, a surveillance of patients with PI, especially those with risk factors, a previous history of OSCC or presence of leukoplakia or lichen planus, is highly recommended, and a biopsy should be performed of lesions that are similar to PI but do not respond well to regular treatment, have a sluggish or rapid progression, or are accompanied by local anesthesia or paresthesia.