INTRODUCTION
Alveolar soft part sarcoma (ASPS), a slow growing and rare malignancy of the mesenchymal connective tissue accounts for 0.4 % to 1 % of all soft tissue sarcomas with a high female predilection. This rare lesion has a proclivity towards age group 15-35 years. It mostly occurs in the lower extremities (44 %), followed by head and neck region (27 %) with a predilection for orbital region and tongue in the younger age group1. Metastatic presentation of this lesion often occurs via the bloodstream due to the increased propensity for vascular invasion. The most common site of metastasis is to the lungs (42-65 %), followed by brain and bone2. In this paper, we discuss a rare case of alveolar soft part sarcoma of the tongue and emphasizing its features on clinical, imaging, and immunohistochemistry which leads us in accurate diagnosis and management.
CASE REPORT
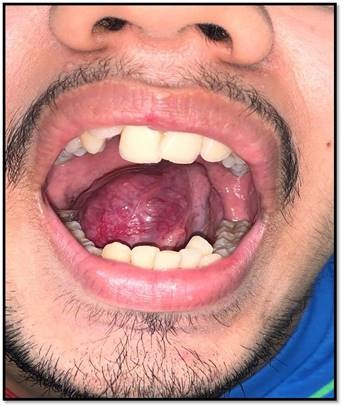
A 19-year-old male patient presented with a 6 months history of a slow growing mass over the right side of his tongue (Figure 1). The lesion was small in size initially and had grown to reach its current size at the time of the visit causing difficulty in speech, swallowing and breathlessness while in supine position. On examination, the lesion was firm, non-tender, measuring approximately 4*3 cm in size, non-fluctuant over the right side of the ventral aspect of the tongue. The mucosa over the swelling was erythematous and presented a tortuous course of capillaries. No significant cervical lymphadenopathy was seen which was confirmed on palpation. A clinical diagnosis of a benign tumour of tongue was considered. The patient advised to undergo a Magnetic Resonance Imaging (MRI) scans. MRI revealed a hyper intense, well circumscribed mass measuring 4*4 cm in size with a central low signal intensity representing an area of tumour necrosis and no sign of feeder vessels (Figure 2A and Figure 2B). Hence, vascular tumours were ruled out. Fine Needle Aspiration Cytology (FNAC) yielded blood aspirate and was non-contributory. We then proceeded with the plan of local excision of the benign lesion under general anaesthesia followed by histopathological examination. The surgical excision was done with a margin of normal tissue three dimensionally and closed primarily with 3-0 vicryl. The patient had an uneventful recovery and was started on oral feeds on post-operative day 1.

Figure 2 A: sagittal Section of MRI exhibiting the circumscribed mass involving the tongue. B: axial section section of MRI showing the circumscribed mass involving the tongue
Histologically the tumour was predominantly arranged as nests, alveolar and organoid pattern with large polygonal cells having abundant eosinophilic to granular cytoplasm with loss of cellular cohesion resulting in pseudo alveolar pattern (Figure 3). Periodic Acid Schiff (PAS) stain showed PAS positive and diastase resistant granules in occasional cells. Immunohistochemistry (IHC) performed revealed strong nuclear positivity in neoplastic cells for TFE3, while negative for Cytokeratin, Vimentin, S-100, Desmin, SMA, HMB45, Synaptophysin, Myo-D1 and EMA. The above findings were consistent with that of Alveolar soft part sarcoma (Figure 4) (Table 1).

Figure 3. Histopathological exhibiting uniform, organoid nests of polygonal tumour cells, separated by fibrovascular septa and delicate capillary-sized vascular channels and a prominent loss of cellular cohesion, leading to the unique pseudo alveolar pattern, H & E, 10x
Subsequently, PET scan was performed post excision to rule out the metastatic evidence in lungs, brain or liver. The PET CT impression reported patchy peripheral metabolic activity on the tongue which may represent post biopsy changes. The patient was advised for a re-surgical procedure of compartmental resection with a radial forearm free flap reconstruction. The patient and his guardian denied re-intervention or any further lines of treatment. Informed consent was obtained stating the need for treatment and high chances of distant spread if left untreated. The patient is devoid of his complaints currently and is recovering uneventfully and is under regular follow up.
DISCUSSION
ASPS is a rare type of sarcoma which usually affects people in the first few decades of life. In 1952, It is differentiated from other bony sarcomas and are named after their histological appearance3. The lesion is commonly seen occurring in Extremities, particularly lower limbs4,5. In the head and neck area, particularly the tongue and orbit are known to common sites of involvement. ASPS often has an insidious, slow growth pattern with a long clinical history and a large mass, this pattern was similar to our case, where the patient reported only after he started to have dysphagia.
Lymph node involvement is uncommon and is reported only in 10 % of the patients. The asymptomatic nature, indolent course and their resemblance to benign vascular conditions, both clinically and radiologically often leads to misdiagnosis and delay in the treatment strategy. Aitken and Stone reported a case of ASPS of the tongue which mimicked hemangioma on CT and MRI6. In our case, FNAC was explained to the patient attender, since we explained the chances of it being a vascular tumor due to the clinical picture. Attender was against doing a needle test and wanted the biopsy procedure. TNM staging done of the tumor was concluded to be at T1 with N0 and M0 and a staging at 1A.
ASPS is histopathologically characterized by uniform, organoid nests of polygonal tumour cells, separated by fibrovascular septa and delicate capillary-sized vascular channels. Within these nests, there is prominent loss of cellular cohesion, leading to the unique pseudo alveolar pattern, after which the lesion has been named. The organoid appearance may be completely lost and the tumour may be composed of sheets of epithelioid cells. Tumours occurring in younger patients and in confined locations, such as the tongue, often show very small nests of cells, closely mimicking true paragangliomas7. The differential diagnosis includes tumours having large cells arranged as nests with eosinophilic/clear cytoplasm. These tumours are malignant melanoma, renal cell carcinoma, granular cell tumour (GCT), paraganglioma, alveolar rhabdomyosarcoma and ectopic lingual thyroid. Renal cell carcinomas, adrenal cortical carcinomas and hepatocellular carcinomas may mimic ASPS by virtue of their abundant eosinophilic to clear cytoplasm.
Immunohistochemical evidence plays a strong role in the diagnosis of ASPS. ASPS are negative for epithelial markers, neuronal markers and melanocytic markers. In such conflicting cases RT-PCR for the fusion transcript is a more suitable method. In our case, Immunohistochemical analysis for TFE3 showed a strong nuclear positivity, which confirmed a diagnosis of ASPS.
It has recently been discovered that ASPS are characterised by a tumour-specific der (17) t(X; 17) (p11; q25) that fuses the transcription factor 3 (TFE3) gene at Xp11 to the ASPL gene at 17q25, creating an ASPL-TFE3 fusion protein8,9. Antibodies directed against the C-terminus of the TFE3 has emerged as a highly sensitive and specific marker of the ASPS. Nuclear expression of TFE3 is of diagnostic value as the antibody binds to the protein downstream of exon 4 and therefore is positive in both fusion variants seen in ASPS.
The best treatment option of ASPS to prevent local recurrence is surgical excision with a tumour free - margin of 1.0 to 1.5 cm to ensure a tumour free margin (R0). The closest surgical margin, which was microscopically defined as positive (tumour within 1 mm of the inked surface, R1) or negative (tumour greater than 1 mm from the inked surface, R2), was used to classify all macroscopically complete resections (absence of tumour within 1 mm from the inked surface, R0). The influencing prognosis of the surgical margin of alveolar soft tissue sarcoma is known from many earlier reports, and most tumour specialists feel that it is crucial for the complete recovery of a local tumour. En-bloc tumour removal with a sufficient number of normal cells surrounding the tumour should be doable for this. The smaller the tumour, the less likely it is to be included in the surgical margin en-bloc, hence tumour size can affect surgical margin. The presence of tumour cells in the surgical margin indicates that active tumour cells have survived and will require more intensive therapy. Chemotherapy's role in treatment is debatable. Radiotherapy is usually given to patients for residual lesions after surgical excision and for metastatic conditions10. The prognosis of ASPS is largely dependent on location of the primary lesion, age of the patient, size of the tumour and presence of metastasis on diagnosis. The prognosis for children may be considerably better; lingual and orbital tumours also have very high survival rates, possibly reflecting a combination of small size at the time of diagnosis and younger patient age.
CONCLUSION
The clinical presentation and microscopic appearance of ASPS is variable and mimics benign lesions on many occasions. Histopathological examination along with immunohistochemistry and special stains helps in confirming the diagnosis. Treatment is primarily surgical excision with limited roles of adjuvant chemotherapy and radiotherapy. Early diagnosis and treatment with routine follow up is absolutely crucial for a better prognosis.