Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.38 no.2 Toledo mar./abr. 2014
https://dx.doi.org/10.7399/FH.2014.38.2.1125
A better regulation is required in viral hepatitis smartphone applications
Necesidad de una mayor regulación en aplicaciones para smartphone sobre hepatitis virales
M.a R. Cantudo-Cuenca1, M.a A. Robustillo-Cortés1, M.a D. Cantudo-Cuenca2 and R. Morillo-Verdugo
1 Pharmacy Departament Área de Gestión Sanitaria Sur Sevilla. Seville. Spain.
2 Pharmacy Departament. Complejo Hospitalario. Jaén. Spain.
ABSTRACT
Aim: To describe the characteristics and content of the available viral hepatitis mobile applications, as well as assess the level of participation of medical professionals in their development.
Methods: A descriptive observational study was carried out in September 2013. We searched smartphone apps specifically relating to the viral hepatitis for using a keyword search with the following terms; "hepatitis", "hepatology", "hbv" and "hcv" in the Google Play Store (Android) and the Apple App Store (iOS). Data recorded included: name, platform, category, cost, user star rating, number of downloads, date the app was updated by the developer and target audience. We analysed the content of the applications, and these were then categorised based on the viral hepatitis type into three groups. We conducted an analysis in which we specifically examined the authorship in order to assess the prevalence of health professional participation in their development.
Results: A total of 33 apps were included (from 232 that were identified), among which there were 10 duplicates. Most of these apps were uploaded under the medical category. Three had ratings less than 3.9 stars (out of 5). Only 6 apps had exceeded 1000 downloads. A total of 12 apps were aimed at health professionals, while 4 focused on patients (7 for both of them). The participation of health professionals in the development of apps was 56.6%.
Conclusions: Viral hepatitis apps are available for both professionals and patients; however, much of the information contained within them is often not validated. They should be certificated.
Key words: Cellular phone: Hepatitis; Viral; Human; Internet; Medical Informatics Applications; Telemedicine.
RESUMEN
Objetivo: Describir las características y el contenido de aplicaciones móviles disponibles sobre hepatitis vírica, así como el nivel de participación de los profesionales médicos en su desarrollo.
Métodos: Se realizó un estudio observacional descriptivo en septiembre de 2013. Buscamos en la tienda Google Play (Android) y en la tienda Apple App (iOS) aplicaciones para teléfonos inteligentes que se relacionasen específicamente con la hepatitis vírica empleando una búsqueda por palabras claves que incluía los siguientes términos: 'hepatitis', 'hepatología', "HBV" y 'HCV'. Los datos recogidos incluían: nombre, plataforma, categoría, coste, puntuación del usuario por estrellas, número de descargas, fecha en la que el creador actualizó la aplicación y público objetivo. Analizamos el contenido de las aplicaciones y se distribuyeron en 3 categorías en función del tipo de hepatitis vírica. Realizamos un análisis en el que se examinó específicamente la autoría con el fin de evaluar la prevalencia de la participación de los profesionales sanitarios en su desarrollo.
Resultados: Se incluyó un total de 33 aplicaciones (de 232 identificadas), de las cuales 10 estaban duplicadas. La mayoría de las aplicaciones se subían en la categoría médica. Tres tuvieron puntuaciones menores de 3,9 estrellas (de 5 posibles). Sólo 6 aplicaciones superaban las 1000 descargas. Un total de 12 aplicaciones estaban dirigidas a profesionales sanitarios mientras que 4 se centraban en los pacientes (7 para ambos colectivos). La participación de los profesionales sanitarios en el desarrollo de las aplicaciones fue del 56,6%.
Conclusiones: Existen aplicaciones disponibles sobre hepatitis vírica tanto para profesionales sanitarios como para pacientes; sin embargo, la mayor parte de la información contenida en ellas a menudo no está validada. Deberían estar certificadas.
Palabras clave: Cellular Phone; Hepatitis; Viral; Human, Internet; Medical Informatics Applications; Telemedicine.
Introduction
Advancements in mobile technology, along with the everywhere of the smartphone, have had a profound effect on the practice of medicine. In the last years, the smartphone has been one of the most prosperous inventions,1 revolutionising and facilitating delivery of care in medicine.2 Smartphones integrate a system that is able to perform multiple tasks,3 making their use more all-around than that of a simple mobile telephone.2 Most smartphones can be connect to other devices2 and also integrate audio and video, internet access, a touchscreen display and the ability to download and execute applications called "apps" in a pocket device,3 creating a cheap and portable tool. Each technology and their combined capabilities can be used in different apps for our health professional lives.2
Smartphone is characterised by its operating system (OS), and the currently available devices run one of Windows, Nokia, Blackberry, Apple, and Android. These mobile platforms are the most popular as evidenced by device sales in 2011. The latter two platforms represent the majority of the smartphone market. Google Android ranked as the top smartphone platform with 43.7% market share, whereas Apple strengthened its second position, with 27.3% of the smartphone market.4 Smartphones are already a popular option of mobile telephone among health professionals. In recent years, smartphone and mobile app use among health care providers has mirrored that of the general population.5 The percentage of health professionals using smartphones for accessing medical information has risen in few years.6 As 2011, an estimated 38% of physicians with smartphones used medical related apps on a daily basis.7 The number of mobile health applications has also grown dramatically over the past few years. To date, there are 10000 apps available in the "medical category" of Apple's iTunes App Store and over 3000 on Android Google Play Store.8,9 Since the platforms facilitate development and distribution of mobile apps by clinicians and other developers, rapid proliferation of the market will probably continue.10
Chronic disease constitutes a fast increasing burden to society. The World Health Organization estimates that 46% of global disease is due to chronic diseases.11 Hepatitis virus infection is a major health problem worldwide. For hepatologists worldwide, there have never been more challenges faced, yet never more tools available to overcome them. Estimated numbers of hepatitis B virus (HBV) and hepatitis C virus (HCV) infected subjects worldwide are staggering (over 370 and 130 million subjects respectively).12 Around 80% of the world's population live in areas with mobile phone coverage, making mobile technology probably the most viable type of technology to reach the majority of the world's population.13 Mobile technology presents an opportunity for health-workers to continue to provide the lead in the development of medical technology. Therefore, it can play an important performance in viral hepatitis education. With smartphone use becoming more widespread, the medical community has embraced this technology with a number of apps already available to patients with viral hepatitis infection assisting. Actually, health professionals are prescribing apps to manage health problems.14,15 Many health interventions with mobile phones have been designed to facilitate test result notification16 and improve medication adherence.17 So smartphone apps can increase efficiency within medical practice and provide constantly updated clinical evidence. Nevertheless, recent studies have addressed the lack of evidence and professional medical involvement in their design and development, raising concerns regarding the reliability and accuracy of their medical content, and the consequences for patient safety.18
The current study was designed to describe the characteristics and content of the available viral hepatitis mobile applications, as well as assess the level of participation of medical professionals in their development.
Methods
A descriptive observational study was carried out in September 2013. We searched smartphone apps specifically relating to the viral hepatitis for using a keyword search with the following terms; "hepatitis", "hepatology", "hbv" and "hcv" in the Google Play Store (Android) and the Apple App Store (iOS). App descriptions and available screenshots as provided by site were analysed. All smartphone or tablet apps with viral hepatitis content, targeted at patients or healthcare professionals, were included. A list of all identified apps to download was compiled. Apple apps were downloaded onto an iPhone 4 and an iPad 2 tablet, while Android apps were downloaded onto a Samsung Galaxy II. If an app had a free and a fee-based version, both versions were downloaded. We excluded an app if it did not feature viral hepatitis information or was not available in English.
Data recorded for every app included: name, platform (iOS, Android, or both), category, cost, number of customer downloads (available for Android only), user star rating, the date the app was updated by the developer. These variables were obtained from the official app stores. After download, we tested each eligible app and additional information was also recorded for each app; target audience, type of viral hepatitis, authorship, and health profesional involvement in their design.
The content of each app was analysed by two authors that worked independently of each other. The apps were then categorised based on the viral hepatitis type (B, C or all of them). In addition, they were classified based on the target audience into the following categories: patients or healthcare workers. Overlap in the two categories was allowed if the app catered to patients and healthcare workers. The patients group included apps that contained viral hepatitis information that was considered (by the researchers) to be useful for patients (eg, prevention of disease, selfmonitoring of symptoms, alarm, drug side effects, and so on). The second group included apps with scientific and clinical information that could be considered useful for healthcare professionals (eg, updated guidelines, drug interactions, treatments, news, scientific articles, and so on). We used a standard questionnaire for each app. Each reviewer recorded their responses and these were compared. Any discrepancies were resolved by a third researcher.
An analysis of our selected smartphone apps was conducted in which we specifically examined the authorship in order to gauge the prevalence of health professional involvement in their development and content. We checked if the app indicated who the authors of the content were, and whether the app provided information about their qualifications. The developers of apps were contacted if did not specify this information either in the app or the app store. To evaluate health professional involvement, apps were classified based on the developing agency: (1) healthcare organisations (such as medical associations, hospitals, research associations, public health organizations and medical journals) or (2) non-healthcare associated (ie, any uploading agency which did not fall in the above category).
Results were tabulated using Excel 2007 (Microsoft, Redmond, Washington, USA). Descriptive statistics were used to summarise the results of the content assessment. We analysed averages and proportions. Inter-rater variability was measured with Cohen's kappa. All statistical analysis was performed using SPSS Statistics V.20.0 for Windows (SPSS Inc.).
Results
We identified a total of 232 apps via the keyword search. After screening the store-provided app descriptions, 45 eligible apps were downloaded for full review. We excluded eight apps that did not include viral hepatitis information and four apps not available in English (three, in Spanish, and one, in French). All analysed apps are summarised in table 1. In all, 16 apps were identified on the Google Play Store (Android) and 17 apps on the Apple App Store (iOS). Of these 33 apps, 10 were duplicated on both sites. We found one tablet-only app ("Hepaxpert"); the others iPhone apps could run on the iPad tablet.
The characteristics of the apps are further summarised in table 2. Most (69.6%) of the viral hepatitis apps were uploaded under the medical category, whereas 17.4% were classified under the health and fitness category. The mean cost of apps in the present study was €4.96, but after excluding the 14 apps that were free of charge, the average cost for paid apps was €11,89 (range: €0.69-89.99).
Based on reported number of downloads, the most popular app was "inPractice® Hepatology" that had between 5000-10000 downloads. Of the 23 apps, 12 (52.2%) had customer satisfaction ratings. Of these app reviews, three were from single customers (ie, only 1 review) and only an app had more than 15 reviews. A total of 11 (91.7%) apps with customer satisfaction ratings were free and three (27.3%) without ratings were also free. "inPractice® Hepatology" was the app with the most ratings (17 raters and a mean rating of 4.4 stars out of a possible 5). This app is available free of charge and provides insight and practical recommendations for the care of patients with viral hepatitis. It was developed by USF Health at the University of South Florida.19 There was an average store rating of 4.1 stars, with the highest rated apps being "Prometheus" (5 stars). Prometheus is a web based tool to estimate the likelihood of Hepatitis C Virus cure before initiating therapy in HIV-HCV coinfected patients.20 Only six apps had exceeded 1000 downloads.
Among the list of viral hepatitis apps studied, 11 (47.8%) had been released or updated in the period September 2012 to September 2013, and three had been updated within the last month.
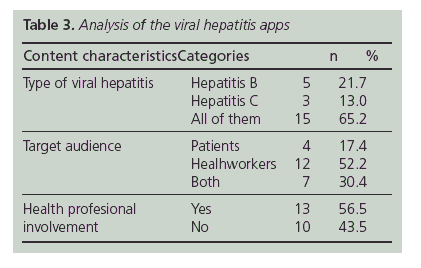
Table 3 describes the content analysis of the apps. Five apps included information exclusively about viral hepatitis B. Based on user ratings and reviews, these apps were considered useful (three apps had a mean rating of 5 stars and the others were unrated). One of them ("Heal-thy B") provides medication and visit reminders and HBV DNA and ALT tracking, as well as, general information about viral hepatitis B and their treament. "HepBaware" and "HCC risk calculator" were designed to predict the risk of getting Hepatitis B when travelling abroad and the risk for hepatocellular carcinoma in patients with chronic hepatitis B. Only two hepatitis B apps (out of five) had named health professional s participation. All apps related with hepatitis C had the participation of a named medical professional. The "Answersln Medicine Hepatitis C" app contains videos about clinical features, diagnosis and treatment of hepatitis C infection. The "Hepatitis C Guidelines" provides evidence-based treatment guidelines based on the latest American Association for the Study of Liver Diseases (AASLD) guidelines. More than half of the apps (53.3%) belonging to the "all of type of viral hepatitis" category had named health professional s involvement. In all, 17.4% of apps provided news and articles about viral hepatitis. A small number of apps ("HEP iChart" and "Viral Hepatitis-A Hepatitis Virus Point of Care Reference") provided a component to look for drug interactions. "Hepatitis connect" was designed to offer support for patients and family members of patients diagnosed with viral hepatitis infection. Interrater agreement for content areas was 100% (Cohen's kappa = 1).
Discussion
The present study documents the variety of viral hepatitis related mobile apps designed for patients and/or health providers. It was considered that the four key words: "hepatitis", "hepatology", "hbv" and "hcv", should source most of the viral hepatitis apps. However, these key word searches yielded a large proportion of apps not relevant to those seeking apps related to viral hepatitis, and they were excluded. To date, the number of these apps apps is limited in relationship to the total number of apps related to the health.21 The present study shows limited downloads and low numbers of user reviews. Although viral hepatitis-related apps have a relatively small number of reviews in comparison with other health apps, these reviews provide a rough reflection of app use; however, they are likely an underestimation, because app reviews represent only a fraction of the total downloads. In addition, almost half of those apps have not had medical professional participation in their development. It is important that users recognize the potential risks of using mobile apps as resources for valid viral hepatitis information. Recent studies have addressed the lack of evidence and professional health participation in their development, raising concerns regarding the reliability and accuracy of their medical content, and the consequences for patient safety.22,23
In spite of the need for qualified health professionals or public organizations to be involved in the regulation of health apps,24,25 the conclusions of our study have obviously demonstrated that this is usually not the instance. Some studies of mobile apps within other specialties have found a profound lack of academic reference or authorship by medical professionals. Rosser et al.26 showed almost the same findings in a review of generic condition of pain apps. Eighty-six percent of 111-reviewed pain-management apps were found to have no medical professional involvement. In relationship with cancer-related apps, there was a lack of these apps with scientifically backed data.6 O'Neill et al.2 found there was little medical professional involvement in the design of colorectal diseases apps. Visvanathan et al.27 showed that smartphones and apps possess many potential uses within microbiology, but more robust regulatory process may be required to prevent future harm to patients. In other specialties such as dermatology28 and HIV29 reports have surfaced. They have shown a shortage of academic reference and low level of health professional participation in app development.28,29 In an attempt to evaluate apps available to cardiothoracic surgery trainees, Edlin et al2 have shown that regulation is missing. They advise that apps be used with caution until systems of peer review and regulation are in place.30
The limitations of this study relate to the app stores. Apple App Store and the Android Google Play Store account the most of the global app market, but there are other smartphone app stores for Windows Phone, Blackberry and Samsung. The ratings given are subjective and not independent. The number of downloads were only available for six apps (in the Google Play Store). Depending on current popularity, the prices of apps commonly vary. In addition, by the time of publication, some apps will have been added, while others will have been removed, so the number of viral hepatitis apps usually changes.
New mobile apps are developed daily, offering a wide array of tools for health care professionals, as well as for the general public. However, the use of smartphones for health professionals and patients is not without concern. It has been proposed that medical apps should be peer-reviewed by clinical experts and that regulatory measures should be increased in order to safeguard quality of care. Regulation and guidance are urgently needed.24 There is, at present, no way to regulate the content or validity of the information. Instead, smartphone users must independently verify the information provided. Medical professionals must be made aware that some apps contain unreliable, non-peer-reviewed content so that they can choose carefully which apps to use in clinical care. Recently, the American Food and Drug Administration (FDA) published a draft guideline on how to regulate medical apps.30 The FDA plans to actively regulate certain types of apps. This is a positive development. Nevertheless, at the same time, government health authorities should not over-regulate medical apps so as to retain their open nature. The regulation process should be managed primarily by the healthcare community itself. However, it would be beneficial for government health authorities to provide official certification marks guaranteeing the quality of apps so that physicians can make an informed choice as to whether an app has evidence-based reliability.
There are many advantages to viral hepatitis apps as tools to obtain medical information that can be accessed anywhere at any time via a smartphone. These apps are available for both professionals and patients; however, much of the information contained within them is often not validated. Apps should be certificated to allow clinicians to direct patients to appropriate and useful apps.
Conflict of interest
The authors report no financial conflicts of interest related to the subjects discussed in this article.
Bibliography
1. Boulos MN, Wheeler S, Tavares C, et al. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online. 2011; 10:24. [ Links ]
2. Edlin JC, Deshpande RP. Caveats of smartphone applications for the cardiothoracic trainee. J Thorac Cardiovasc Surg. 2013;146(6): 1321-6. [ Links ]
3. O'Neill S, Brady RR. Colorectal smartphone apps: opportunities and risks. Colorectal Dis. 2012;14:530-4. [ Links ]
4. ComScore MobiLens Service. ComScore reports August 2011 U.S. mobile subscriber market share. Available at: www.comscore.com/Insights/Press_Releases/2011/10/comScore_Reports_August_2011_U.S._Mobile_Subscriber_Market_Share. Accessed March 10, 2013. [ Links ]
5. Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149(11):1300-4. [ Links ]
6. Pandey A, Hasan S, Dubey D, et al. Smartphone Apps as a Source of Cancer Information: Changing Trends in Health Information-Seeking Behavior. J Cancer Educ. 2013;28:138-42. [ Links ]
7. CompTIA. Healthcare practices embrace mobile technologies, new CompTIA research reveals. Available at: http://www.comptia.org/news/pressreleases/11-11-16/Healthcare_Practices_Embrace_Mobile_Technologies_New_CompTIA_Research_Reveals.aspx. Accessed October 1, 2013. [ Links ]
8. Apple Press Info. Apple's App Store Downloads Top 15 Billion. Available at: http://www.apple.com/pr/library/2011/07/07Apples-App-Store-Downloads-Top-15-Billion.html. Accessed September 2, 2013. [ Links ]
9. AppBrain. Android statistics. Top categories. Available at: www.appbrain.com/stats/android-market-app-categories. Accessed May 9, 2013. [ Links ]
10. Buijink AW, Visser BJ, Marshall L. Medical apps for smartphones: lack of evidence undermines quality and safety. Evid Based Med. 2013;18(3):90-2. [ Links ]
11. Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. J Parenter Enteral Nutr. 2006;30:45-51. [ Links ]
12. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6-9. [ Links ]
13. Vital Wave Consulting. mHealth for Development: The Opportunity of Mobile Technology for Healthcare in the Developing World. Washington, DC and Berkshire, UK: UN. Foundation-Vodafone Foundation Partnership, 2009. Available at: http://unpan1.un.org/intradoc/groups/public/documents/unpan/unpan037268.pdf. Accessed May 13, 2013. [ Links ]
14. Available at: http://www.dispatch.com/content/stories/science/2013/03/31/1-the-doctors-appswill-see-you-now-.html. Accessed July 13, 2013. [ Links ]
15. Most patients want their doctors to prescribe apps. Available at: http://mobihealthnews.com/23418/most-patients-want-their-doctors-to-prescribe-apps/. Accessed September 30, 2013. [ Links ]
16. Lim EJ, Haar J, Morgan J. Can text messaging results reduce time to treatment of Chlamydia trachomatis? Sex Transm Infect. 2008; 84:563-4. [ Links ]
17. Hardy H, Kumar V, Doros G, et al. Randomized controlled trial of a personalized cellular phone reminder system to enhance adherence to antiretroviral therapy. AIDS Patient Care STDS. 2011;25: 153-61. [ Links ]
18. Burdette SD, Herchline TE, Oehler R. Surfing the web: practicing medicine in a technological age: using smartphones in clinical practice. Clin Infect Dis. 2008; 47:1 17-22. [ Links ]
19. inPractice® Hepatology. Available at: https://play.google.com/store/apps/details?id=com.cco.android.inpractice.hepatology&hl=es. Accessed October 1, 2013. [ Links ]
20. Available at: https://play.google.com/store/apps/details?id=lara.oreka.Prometheus&hl=en_GB. Accessed October 1, 2013. [ Links ]
21. Infographic: mHealth apps by the numbers. Available at: http://www.mhealthnews.com/news/infographic-mhealth-apps-numbers. Accessed January 4, 2014. [ Links ]
22. Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12:67. [ Links ]
23. Rodrigues MA, Brady RR. Anaesthetists and apps: content and contamination concerns. Anaesthesia. 2011;66:1172-85. [ Links ]
24. Buijink AW, Visser BJ, Marshall L. Medical apps for smartphones: lack of evidence undermines quality and safety. Evid Based Med. 2013;18(3):90-2. [ Links ]
25. Ferrerò NA, Morrell DS, Burkhart CN. Skin scan: a demonstration of the need for FDA regulation of medical apps on iPhone. J Am Acad Dermatol. 2013;68:515-16. [ Links ]
26. Rosser BA, Eccleston C. Smartphone applications for pain management. J Telemed Telecare. 2011;17:308-12. [ Links ]
27. Visvanathan A, Hamilton A, Brady RR. Smartphone apps in microbiology-is better regulation required? Clin Microbiol Infect. 2012; 18:E218-12. [ Links ]
28. Hamilton AD, Brady RR. Medical professional involvement in smartphone apps in dermatology. Br J Dermatol. 2012;167:220-1. [ Links ]
29. Cantudo Cuenca MR, Cantudo Cuenca MD, Morillo Verdugo R. Availability and medical professional involvement in mobile healthcare applications related to pathophysiology and pharmacotherapy of HIV/AIDS. Eur J Hosp Pharm. 2013;20:356-61. [ Links ]
30. U.S. Food and Drug Administration. Draft Guidance for Industry and Food and Drug Administration Staff-Mobile Medical Applications. Available at: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM263366.pdf. Accessed July 27, 2013. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: rosa_cantudo@hotmail.com
(M.a R. Cantudo-Cuenca).
Recibido el 16 de noviembre de 2013
Aceptado el 13 de febrero de 2014.