Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.39 no.6 Toledo nov./dic. 2015
https://dx.doi.org/10.7399/fh.2015.39.6.8907
REVISIONES
Physiopathology and treatment of critical bleeding: a literature review
Fisiopatología y tratamiento de la hemorragia crítica: una revisión de la literatura
Celia González Guerrero and José Bruno Montoro Ronsano
Servicio de Farmacia, Hospital de la Vall d'Hebrón de Barcelona, Spain.
ABSTRACT
Objectives: to develop the different factors involved in the physiopathology of trauma-induced coagulopathy, through a review of publications on the matter; as well as to assess the evidence available on the treatment of critical bleeding and the recommendations by clinical practice guidelines.
Methods: a search has been conducted on the bibliography published about the physiopathology and treatment of critical bleeding in the PUBMED, BestPractice, UpToDate databases and the Cochrane Plus Library. The main key words used for this search were "early trauma induced coagulopathy", "mechanisms of early trauma-induced coagulopathy", "blood transfusion guidelines", "massive transfusion guidelines" and "fibrinogen replacement therapy". The most clinically relevant articles were selected for this review.
Conclusions: the physiopathology of the trauma-induced coagulopathy is a more complex matter and involves more factors than was initially assumed. The early treatment of the coagulopathy is critical for the initial management of the critical bleeding. However, the use of blood derivatives should be rational and based on homogeneous and high-quality scientific evidence.
The main cornerstones for the treatment of critical bleeding are: fluid therapy, fibrinogen concentrate, prothrombin complex concentrate, plasma, erythrocyte or platelet concentrates, tranexamic acid, and calcium. Their administration should be assessed depending on the clinical condition of each patient.
Key words: Treatment for critical bleeding; Physiopathology of the trauma-induced coagulopathy; Fibrinogen; Prothrombin complex; Blood derivates.
RESUMEN
Objetivos: desarrollar los factores implicados en la fisiopatología de la coagulopatía asociada al traumatismo (CAT) mediante una revisión de la literatura publicada al respecto; además de revisar la evidencia disponible sobre el tratamiento de la hemorragia crítica y las recomendaciones de las guías de práctica clínica.
Métodos: se ha realizado una búsqueda de la bibliografía publicada sobre la fisiopatología y tratamiento de la hemorragia crítica en las bases de datos PUBMED, BestPractice, UpToDate y la Biblioteca Cochrane Plus. Las principales palabras clave utilizadas para la búsqueda han sido: "early trauma induced coagulopathy", "mechanisms of early trauma-induced coagulopathy", "blood transfusión guidelines", "massive transfusion guidelines" y "fibrinogen replacement therapy". Los artículos más clínicamente relevantes han sido seleccionados para la revisión.
Conclusiones: la fisiopatología de la coagulopatía asociada al traumatismo se trata de un cuadro más complejo y multifactorial de lo que inicialmente se había aceptado. El tratamiento precoz de la coagulopatía es imprescindible para el manejo inicial de la hemorragia crítica. No obstante, el uso de hemoderivados debería ser racional y basado en una evidencia científica homogénea y de alta calidad.
Los principales pilares del tratamiento de la hemorragia crítica son la fluidoterapia, el concentrado de fibrinógeno, el concentrado de complejo protrombínico, el plasma, los concentrados de hematíes o de plaquetas, el ácido tranexámico y el calcio. Su administración debería valorarse en función de las condiciones clínicas de cada paciente.
Palabras clave: Tratamiento de la hemorragia crítica; Fisiopatología de la coagulopatía asociada al traumatismo; Fibrinógeno; Complejo de protrombina; Hemoderivados.
Method
A search was conducted on the bibliography published about the physiopathology and treatment of critical bleeding in the PUBMED, BestPractice database, UpToDate database, and the Cochrane Plus Library. The main key words used for this search were: "early trauma induced coagulopathy", "mechanisms of early trauma-induced coagulopathy", "blood transfusion guidelines", "massive transfusion guidelines" and "fibrinogen replacement therapy". The most clinically relevant articles have been selected for this review.
Introduction
Critical bleeding is the main cause of avoidable death after trauma. A fourth of all trauma patients will present a trauma-induced coagulopathy (TIC). Patients with TIC have a five times higher risk of death within the first 24 hours, higher transfusion requirements, a longer hospital stay, and are susceptible to presenting more complications1, Brohi2 and MacLoad3 had already stated in 2003 that trauma itself is the trigger for trauma-induced coagulopathy.
Within the setting of trauma patients, coagulopathies can be classified into two groups: TIC in the strict sense, or iatrogenic TIC. TIC is a pathologic response due to a deregulation of hemostasis, secondary to a trauma injury. Iatrogenic TIC, on the other hand, is caused by previous treatment with oral anticoagulants, or by hemodilution due to an abundant fluid therapy administered after the critical bleeding1.
Paradoxically, there are many similarities between disseminated intravascular coagulation with fibrinolytic phenotype (DIC) and TIC: low levels of fibrinogen (increased fibrinolysis and, therefore, increase in the fibrinogen and fibrin degradation products), low platelet count, prolonged prothrombin time, and low levels of proteins controlling coagulation (e.g. antithrombin levels are lowered, and therefore, hypercoagulation will develop; or there is a reduction in the alpha2-plasmin inhibitor, leading to higher fibrinolysis)4.
Thus, the objectives of the present paper are to develop which mechanisms are involved in the physiopathology of trauma-induced coagulopathy (TIC), through a review of literature published about this matter, as well as to review the evidence available on the treatment for critical bleeding and the recommendations by clinical practice guidelines.
Results
Tic physiopathogenesis
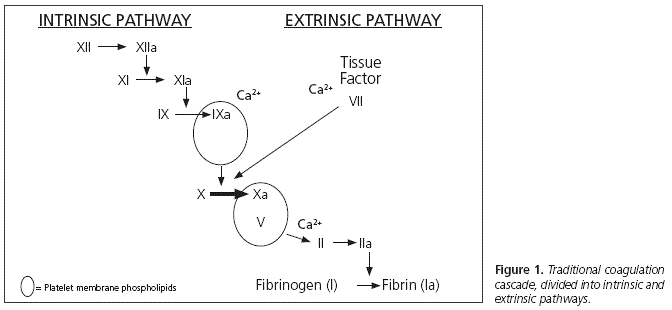
Traditionally, TIC mechanisms were focused on the hemodilution + hypothermia + acidosis triad. Though this triad is still valid, recent studies on the physiopathology of TIC, besides the Cell-based Model5,6, have demonstrated that this is a more complex matter which involves more factors than was initially assumed7 (Figure 1).
Other factors involved:
- Platelet dysfunction: The involvement of the platelet function was considered when sustained bleeding was observed in trauma patients with normal platelet counts. Multiple factors would be involved in platelet dysfunction: hypothermia (a consequence of bleeding and hemorrhagic shock), lesion severity, and the storage methods for platelet concentrates after donation by volunteers (media, temperatures, processing...) It is believed that platelet dysfunction would also be affected as a response, for example, to ADP (adenosine dyphosphate) arachidonic acid, collagen, and thrombin receptor activating peptide1, 8-10.
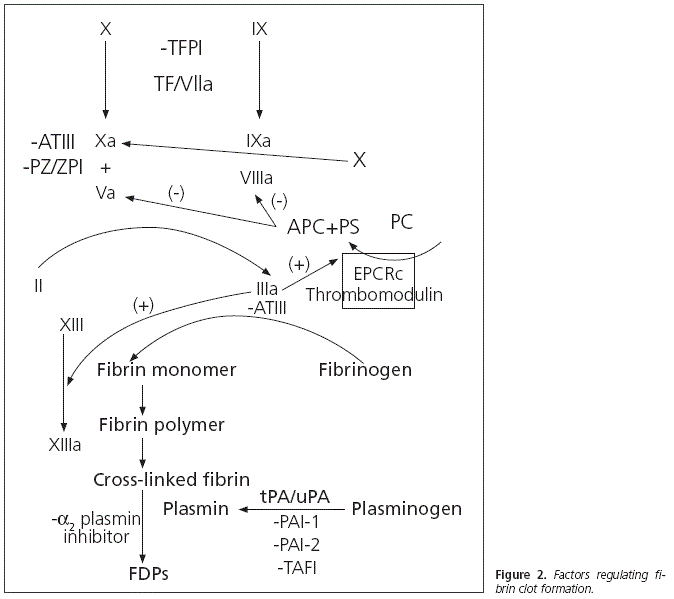
- Endothelial dysfunction: Some endothelial cells generate proteins, in the context of trauma, which will favour patient anticoagulation. These proteins inhibit thrombin formation through the production of thrombomodulin. With the activation of the protein C endothelial receptor, they will also produce chondroitin and heparan sulfate1, as well as a recently studied glycoprotein (sydecan-1)11. Chondroitin sulfate increases the efficiency of the thrombin inhibition conducted by the thrombomodulin; while heparin sulfate increases the efficiency of thrombin inhibition conducted by antithrombin III. Ostrowski et Johansson12 described an endogenous heparinization in 5% of those trauma patients studied, which corresponded to those patients with higher lesion severity, higher transfusion requirements, more prolonged prothrombin times, and higher evidence of endothelial damage.
- Protein C activation: Protein C plays a key role in TIC development. It presents a dual activity: cytoprotective and antiacogulant. On one hand, cytoprotective against cytotoxicity secondary to hypoperfusion after hemorrhagic shock, antiinflammatory and limiting endothelial permeability (higher permeability, higher inflammation and higher oedema); and on the other hand, anticoagulant inhibiting thrombin formation (inhibiting the FVa and FVIIIa factors) and promoting fibrinolysis (encouraging plasmin formation and inhibiting PAI-1, the physiological inhibitor of tPA and uPA, thus encouraging fibrin clot degradation and increasing the levels of fibrinogen degradation products)1, 13,14.
- Oxidative modification of proteins involved in coagulation: It is believed that the oxidative modification of certain domains of proteins involved in coagulation, such as PAI-1, C protein, thrombomodulin or fibrin, would encourage TIC development15,16. Burney et al.17 have recently provided data about the oxidation of a methionine located in the alfa C-subdomain of fibrin. This results in the alteration of the fibrin lateral aggregation during polymerization, with the subsequent involvement of compact clot formation. This oxidative damage would appear in the setting of oxidative stress, such as hemorrhagic shock secondary to trauma. Reactive oxygen species are liberated by leukocytes, platelets and endothelial cells after the activation of inflammatory pathways, endothelial lesion and tissue hypoperfusion.
- Hyperfibrinolysis: The vast majority of multiple trauma patients will present a certain degree of fibrinolysis, and approximately 5% (according to Raza et al.18) would present severe hyperfibrinolysis. Fibrinogen degradation is controlled by plasmin, which is generated through plasminogen activation by tPA and uPA. Plasmin will degrade the cross-links between fibrin molecules, dissolving the fibrinogen clot. This fibrinogen degradation process is inhibited by PAI-1, which deactivates tPA and uPA. The relationship between activated protein C, which inhibits PAI-1, and hyperfibrinolysis, is reinforced. On the other hand, Lustenberger et al.19 have recently studied the TAFI (thrombinactivatable fibrinolysis inhibitor). This is a plasminogen activation inhibitor, and it has been observed to be reduced in patients with TIC. TAFI is activated by thrombin, and inhibits fibrinolysis by splitting a carboxy-terminal end of a lysine residue of the fibrin molecule to which plasminogen and tPA will bind (figure 2).
Critical bleeding treatment
Early treatment for coagulopathy, together with fast diagnosis and the control of the source of bleeding, are three key points in the initial management of critical bleeding. The initial replacement strategy consists essentially in the administration of fibrinogen, prothrombin complex (PCC) and fresh frozen plasma (FFP).
Fibrinogen
This is a glycoprotein synthesized in the liver, necessary both for platelet aggregation and fibrin formation. The conversion of fibrinogen to fibrin is catalyzed by thrombin, and fibrinogen levels will determine the quantity and complexity of the fibrin net formed during coagulation. If fibrinogen levels are reduced, the fibrin net will be more fragile and unstable, and will affect secondary hemostasis20-28.
On one hand, fibrinogen plays a key role in thrombus formation and stabilization; while it will also cause platelet activation and aggregation by binding with the GPIIb/ Ilia platelet receptors. Low levels of fibrinogen have been associated with an increased risk of bleeding and a higher risk of mortality20-28.
Fibrinogen is the first plasmatic factor to become depleted in critical bleeding. There are three ways to supply fibrinogen: fresh frozen plasma, cryopredpltate, and fibrinogen concentrate. The latter is the most frequently used, because it does not need refrigeration, does not require cross-tests, it does not cause hemodilution, and can be administered rapidly (up to 6g in under 3 minutes)26, 27.
Though clinical guidelines recommend fibrinogen administration in order to reduce bleeding and/or transfusion rate (level 1C)26,27,34, the indication according to label for fibrinogen concentrate administration is only for treatment of bleeding in patients with congenital hypo or afibrinogenemia with tendency to bleeding29.
There is no universal consensus regarding the critical levels of fibrinogen for trauma patients, though the key role of fibrinogen in critical bleeding control is widely accepted. Low fibrinogen levels are a negative prognostic factor, and therefore the early correction of levels has been associated with a higher survival. English30,31 and American32 guidelines recommend fibrinogen administration when levels are below 1g/L. On the other hand, the European Trauma Guidelines34, the European Society of Anaesthesiology35 and the Canadian National Advisory Committee on Blood and Blood Products33 recommend maintaining fibrinogen levels above 1.5-2g/L.
Prothrombin Complex Concentrate (PCC)
Those PCCs marketed in Spain contain 4 coagulation factors (II, VII, IX and X). In order to minimize thrombogenicity, they also contain protein C, protein S, antithrombin III and/or heparin. The different commercial brands are equally potent in terms of activity, but they have certain differences in terms of composition. Typically prescribed doses refer to factor IX, and are usually dosed at a mean 20UI/Kg (15-25UI/Kg)26,27,34,38.
Though the indications approved in the product specifications38 for PCCs are urgent reversal of anticoagulation by vitamin K antagonists (normalization of INR between 10 and 30 minutes after administration) and perioperative treatment and prevention of bleeding by congenital deficiency of any of the coagulation factors depending on vitamin K, its off-label use has become increasingly common in non-anticoagulated patients, trauma patients, or those who present uncontrolled bleeding during surgery36-37. In fact, clinical guidelines recommend its use in patients not treated with vitamin K antagonists (VKAs), with coagulopathy in the context of trauma, perisurgical bleeding, or acute liver impairment with a level 2C of evidence / recommendation26,27,34.
An increasing number of clinical guidelines are highlighting its numerous advantages vs. plasma. This is a concentrated product which does not worsen hemodilution or has any impact on the fluid balance of the patient. It can be stored at room temperature, and therefore does not require to be defrosted before administration. It can be administered regardless of blood type, with rapid administration and anticoagulation reversal. In intracranial hemorrhage, which is the most severe event associated with anticoagulation with vitamin K antagonists, studies have demonstrated a higher INR correction and bleeding control in patients treated with PCC vs. those treated with plasma39,40.
Regarding the reversal of new oral anticoagulants, neither clinical guidelines nor product specifications have formally recommended its use, because efficacy and safety data are still very limited. As there is some experimental evidence which seems to support the use of activated prothrombin complex concentrates (e.g. FEIBA®), recombinant Factor VIIa or PCCs, their use could be considered in cases where there is an urgent need to reverse anticoagulation, such as, for example, an emergency surgical procedure41-43.
Plasma
Clinical guidelines recommend (level 1B) an initial dose of 10-15ml/Kg for initial management of critical bleeding26,27,34. Plasma contains both procoagulants (such as coagulation factors and fibrinogen) and coagulation cascade inhibitors; as well as other proteins such as albumin or immunoglobulins. But as has been previously mentioned, it presents certain disadvantages when compared with the fibrinogen concentrate or PCC separately. There are no special storage conditions for any of both concentrates, there is no need to defrost, no compatibility tests are required, they can be administered rapidly with the subsequent rapid effect, and they do not cause the hemodilution generated by plasma. Reviews on the clinical utility of plasma, and studies comparing its use vs. fibrinogen concentrate or PCCs, have questioned the use of plasma for the management of critical bleeding, due to the advantages presented by the latter products44-46.
Erythrocyte and Platelet Concentrates
The administration of Erythrocyte Concentrates (ECs) and Platelet Concentrates will be mostly conducted based on test results. It is not recommended to use hematocrit as an isolated marker for critical bleeding (level 1B). However, the administration of erythrocyte concentrates is recommended in order to maintain hemoglobin levels between 70 and 90mg/L (level 1C)26,27-34.
Primary hemostasis is well preserved with platelet levels of 100x109 platelets/L, as long as platelet function is adequate. It is recommended to administer platelet concentrates in order to maintain levels above 50 x109 platelets/L (level 1C) to prevent thrombocytopenia from contributing to the bleeding26,27-34. Thrombocytopenia will typically develop after factor deficiency (fibrinogen, prothrombin complex...) and after the clinical development of microvascular hemorrhage.
It is known that erythrocytes are involved in hemostasis by stimulating platelet activation and thrombin generation47, and that hematocrit levels below 30% (Hb 9g/dl) will reduce the hemostatic efficacy of platelets. However, more studies are necessary in order to determine the hematocrit and haemoglobin levels required for controlling a massive bleeding.
Tranexamic Acid (TXA)
European clinical guidelines for management of critical bleeding in trauma patients recommend the use of antifibrinolytics (level 1B) in patients with hyperfibrinolysis26,27,34. In the Update of the Seville Document, treatment with tranexamic acid (TXA) is recommended in order to reduce bleeding and/or transfusion rate in multiple trauma patients with significant bleeding (level 1B)26,27.
Antifibrinolytics include TXA and Epsilon-aminocaproic acid: both are synthetic lysine analogues that competitively inhibit plasminogen binding to lysine residues on the fibrin surface, thus preventing the conversion of plasmino-gen into plasmin. TXA is 10 times more potent than Epsilon-aminocaproic acid. The recommended doses for TXA are 10-15mg/Kg followed by a 1-5mg/kg/h infusion; the Epsilon-aminocaproic acid doses are 100-150mg/Kg followed by a 15mg/Kg/h infusion26,27.
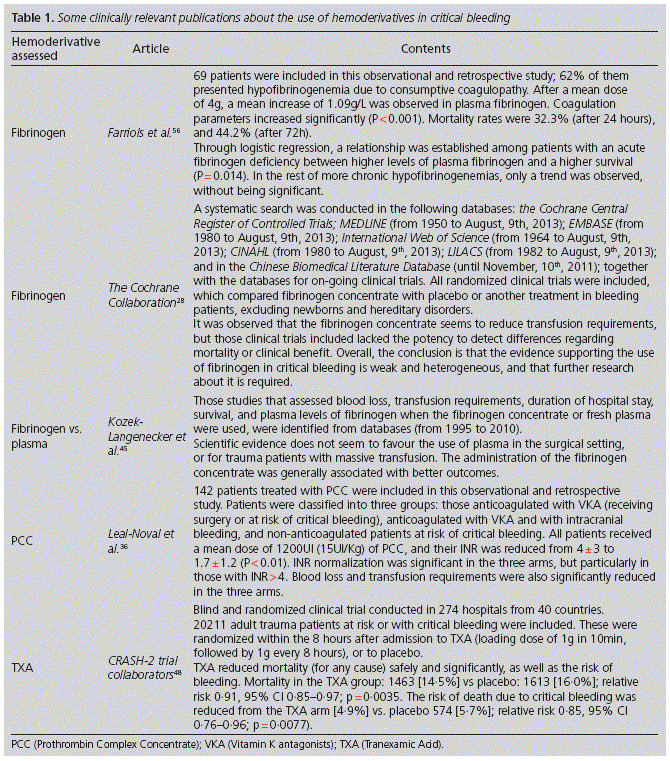
Specifically, TXA is a very cost-effective drug, which has demonstrated in recent studies a reduction in the incidence of coagulopathy and in mortality48,49 (Table 1).
Calcium
Calcium is essential for the activation of coagulation factors in different stages, therefore it is necessary to maintain adequate levels of this cation. Guidelines recommend with an evidence / recommendation level 1C to monitor calcium levels in plasma in order to maintain them >0,9 mmol/l (therapeutic range 1.1-1.3 mmol/l) during massive transfusion. Low levels of plasma calcium at admission have been associated with a higher transfusion requirement and higher mortality. However, there are no data demonstrating that preventing hypocalcemia can reduce mortality among patients at risk of critical bleeding34.
Activated Factor VII (rFVII)
Its authorization in Europe extends to patients with selective factor VII deficiency and Glanzmann thrombasthenia. Its off-label use has been relegated, in patients with bleeding refractory to surgical hemostasis and usual hemotherapy support, as previously mentioned (level 2C)26,27,34. It will only be effective if the sources of active bleeding are controlled, and if the levels of fibrinogen, platelets, hematocrit, plasma calcium, plasma pH, etc., have been minimally normalized34.
A systematic review50 where rFVII was assessed in 5 indications (intracranial hemorrhage, cardiac surgery, trauma, liver transplant, and prostatectomy) concluded that there is no evidence for reduction in mortality with rFVIIa, and that in some of the indications, such as intracranial hemorrhage after traumatic brain injury, it increased the risk of thromboembolism. That it why there is a recommendation against its use in this indication, with a level 2C26,27,34.
Fluid Therapy
Clinical guidelines recommend an early initiation of fluid therapy in those patients with active bleeding and hypotension (level 1A). Hypovolaemia correction through fluid administration is the first measure to take for any type of severe bleeding, because the body has a much lower tolerability to hypovolaemia than to anaemia26,27.
It is recommended that crystalloids should represent the initial option for volemia restoration (level 1B). The most widely used crystalloids are: isotonic saline solution at 0.9%, Ringer's solution, and other "balanced" solutions (Plasmalyte®, containing acetate; Ringer's lactate®, containing lactate). The lactate in the latter can be metabolized into bicarbonate, and in theory could be useful to treat the metabolic acidosis of the lethal triad in these patients; but lactate metabolism is inhibited during shock. On the other hand, Ringer's lactate has been considered a more physiological source of chloride (109mmol/L) than isotonic saline solution at 0.9% (150mmol/L). Some studies51 have even suggested the association between the use of fluid therapy regimens restricted in chloride and a lower incidence of renal impairment and requirement for renal replacement, but results have not been conclusive. That is why, in order to reduce the risk of metabolic disorders during resuscitation or volemic reposition, it is recommended that balanced saline solutions (Ringer's lactate® or acetate) should replace normal saline at 0.9% (level 1B); as long as there is no traumatic brain injury26,27.
The main adverse effect of resuscitation with crystalloids is dilutional coagulopathy. For this reason, when crystalloids are not enough, the use of colloids should be assessed. Those colloids available are: hydroxyethyl starches, gelatins, and human albumin. The disadvantages of gelatins are their low molecular weight, their limited ability to expand (70-80%), and their short half-life (2-3 hours). Albumin has a major volume expanding effect (albumin 5%: 100%; albumin 20%: 200-400%) with fast onset of action and sustained action, but its use is not recommended in the context of the bleeding patient. The problem it presents is that it is not contained exclusively in the intravascular space, and it could worsen the interstitial and pulmonary oedema; at the same time, it could cause coagulation disorders and hemostasis by inhibiting platelet function and increasing the effect of antithrombin III, leading to a state of hypocoagulability. Besides, this is a hemoderivative, with their associated problems (high price, limited source of supply, etc.) Therefore, the solutions that contain hydroxyethyl starches are the most widely used for volume expansion when the single infusion of crystalloids is not considered enough. These must be used at the minimal effective doses and during the shortest period of time possible.
After the safety warnings published in 2013, the European Pharmacovigilance Risk Assessment Committee (PRAC) confirmed that those solutions for intravenous perfusion that contain hydroxyethyl starch must not be used in patients with sepsis, patients in critical condition, or burnt patients, due to a higher risk of severe renal failure and higher mortality. These solutions will only be indicated in case of hypovolemia due to acute bleeding, for a maximum of 24 hours, and watching renal function during at least 90 days, as long as treatment with crystalloid solutions is not considered enough, and taking into account all contraindications and precautions for use52-55.
Thromboelastography (TEG): Bedside management of coagulation
TEG assesses the viscoelastic properties of coagulation during clot formation and lysis. This technique represents an advance in coagulopathy diagnosis, because it allows to make early bedside clinical decisions. In less than 10 minutes, results are obtained graphically, allowing a fast assessment of overall coagulation, and to replace those coagulation factors that the patient is really lacking26,27.
Discussion
It is worthwhile knowing the physiopathology of trauma-induced coagulopathy, because critical bleeding is the main cause of avoidable death after trauma. Besides, in the majority of cases the patient is a healthy individual who could return to their basal situation once they have overcome the coagulopathy, and the trauma if possible. Therefore, it is important also to know in which conditions the use of hemoderivatives will be optimal for critical bleeding management.
Both the hemodilution, hypothermia and acidosis triad used to explain TIC, as the traditional coagulation cascade clearly differentiated between the intrinsic and extrinsic pathways, are very useful from an educational point of view in order to understand the physiopathology of critical bleeding, but they have been gradually relegated. In the new theories, TIC physiopathology would be much more complex, with many more interrelated factors involved.
Undoubtedly, it is also necessary to continue studying which conditions of use are optimal for each hemoderivative, in order not to use them indiscriminately. On one hand, current evidence supporting the use of said hemoderivatives is weak and of low quality. On the other hand, there is a certain wrong perception of the innocuousness of hemoderivatives, regardless of their associated problems. These are not free from adverse reactions (anaphylactic reactions, increased risk of thromboembolism, dilutional coagulopathy, etc.), from a minimal risk of infectious disease transmission, limited sources of supply, and a high economic cost upon hospital budgets.
Conclusions
Trauma-induced coagulopathy is a multifactorial condition, and the components of the coagulation cascade are much more interrelated than was traditionally believed.
Early treatment of coagulopathy is essential for the initial treatment of critical bleeding. However, there should be a rational use of hemoderivatives. The benefit-risk balance should be individually assessed and justified by biochemical tests, blood count test, or thromboelastography.
More studies are required in order to homogenize the recommendations by clinical guidelines, and determine when it will be optimal to use each hemoderivative in the context of trauma-induced critical bleeding.
Bibliography
1. Cardenas JC., Wade CE., et Holcomb JB. Mechanisms of trauma-induced coagulopathy. Curr Opin Hematol 2014, 21:404-409. [ Links ]
2. Brohi K., Singh J., Heron M. et Coats C. Acute traumatic coagulopathy. J Trauma 2003, 54:1127-1130. [ Links ]
3. MackLeod JBA, Lynn M, Mckenney MG, Cohn SM et Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma 2003, 55:39-44. [ Links ]
4. Oshiro, et al. Critical Care 2014, 18:R61 http://ccforum.com/content/18/2/R61. [ Links ]
5. Stephanie A. Smith. The cell-based model of coagulation. Vet Emerg Crit Care 2009;19(1):3-10. [ Links ]
6. Hoffman M., Monroe DM. A cell-based model of Hemostasis. Thromb Haemost 2001;85:958-65. [ Links ]
7. Ross Davenport. Coagulopathy following major trauma hemorrhage: lytic, lethal and a lack of fibrinogen. Critical Care 2014, 18:151 http://ccforum.com/content/18/3/151. [ Links ]
8. Brown LM, Call MS, Margaret Knudson M, et al. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma 2011; 71 (Suppl 3):S337-S342. [ Links ]
9. Wohlauer MV, Moore EE, Thomas S, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg 2012;214:739-746. [ Links ]
10. Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg 2012;73:13-19. [ Links ]
11. Sillesen M., Rasmussen LS, Jin G., et al. Assessment of coagulopathy, endotelial injury, and inflammation after traumatic brain injury and hemorrhage in a porcine model. J Trauma Acute Care Surg 2014;76:12-19. [ Links ]
12. Ostrowski SR., Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg 2012; 73:60-66. [ Links ]
13. Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg 2012;255:379-385. [ Links ]
14. Chesebro BB, Rahn P., Carles M., et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock 2009;32:659-665. [ Links ]
15. Closa D., Folch-Puy E. Oxygen free radicals and the systematic inflammatory response. IUBMB Life 2004;56:185-191. [ Links ]
16. Wood MJ., Helena Prieto J., Komives EA. Structural and functional consequences of methionine oxidation in thrombomodulin. Biochim Biophys Acta 2005;1703:141-147. [ Links ]
17. Burney PR, White N, Pfaendtner J. Structural effects of methionine oxidation on isolated dubdomains of human fibrin D and alphaC regions. PloS One 2014;9:e86981. [ Links ]
18. Raza I., Davenport R., Rourke C., et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost 2013;11:307-314. [ Links ]
19. Lustenberger T., Relja B., Puttkammer B., et al. Activated thrombin-activatable fibrinolysis inhibitor (TAFIa) levels are decreased in patients with trauma-induced coagulopathy. Thromb Res 2013;131:e26-30. [ Links ]
20. Levy JH, et al. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. TRANSFUSION 2014; 54:1389-1405. [ Links ]
21. Schlimp CJ., Schöhl H. The role of fibrinogen in trauma-induced coagulopathy. Hämostaseologie 2014; 34. [ Links ]
22. Massimo Franchini, Giuseppe Lippi. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus 2012; 10: 23-7. [ Links ]
23. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost 2005; 3: 1894-904. [ Links ]
24. Rahe-Meyer N, Sørensen B. Fibrinogen concentrate for management of bleeding. J Thromb Haemost 2011; 9: 1-5. [ Links ]
25. Aubron C, Reade M.C, et al. Efficacy and safety of fibrinogen concentrate in trauma patients- a systematic review. Journal of Critical Care 2014; 29:471.e11-471.e17. [ Links ]
26. Leal-Noval SR, Muñoz M, Asuero M, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the "Seville Document". Blood Transfus 2013; 11: 585-610. [ Links ]
27. Leal-Noval SR, Muñoz M, Asuero M, et al. 2013: Documento "Sevilla" de Consenso sobre Alternativas a la Transfusión de Sangre Alogénica. Farm Hosp. 2012;36(6):209-235. [ Links ]
28. Wikkelsø A, Lunde J, et al. Fibrinogen concentrate in bleeding patients (Review). Cochrane Database Syst Rev. 2013 Aug 29;8:CD008864. [ Links ]
29. AEMPS, Ficha técnica Riastap®, consultada el 23 de Agosto de 2015. http://www.aemps.gob.es/cima/pdfs/es/ft/72725/FT_72725.pdf. [ Links ]
30. Association of Anaesthetists of Great Britain and Ireland, Thomas D, Wee M, Clyburn P, et al. Blood transfusion and the anaesthetist: management of massive haemorrhage. Anaesthesia 2010;65:1153-61. [ Links ]
31. UK Blood Transfusion & Tissue Transplantation Services. The handbook for transfusion medicine. 2007. (cited 2014 August 6). Available from: http://www.transfusionguidelines.org.uk/docs/pdfs/htm_edition-4_all-pages.pdf. [ Links ]
32. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report. Anesthesiology 2006; 105:198-208. [ Links ]
33. National Advisory Committee on Blood and Blood Products. Guidelines for massive transfusion. 2011. (cited 2014 August 6). Available from: http://www.nacblood.ca/resources/guidelines/massive-transfusion.html. [ Links ]
34. Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013;17:R76. [ Links ]
35. Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guideline s from the European Society of Anaesthesiology. Eur J Anaesthesiol 2013;30:270-382. [ Links ]
36. Leal-Noval SR., López-Irizo R., et al. Efficacy of the prothrombin complex concentrate in patients requiring urgent reversal of vitamin K antagonists or presenting with uncontrolled bleeding: a retrospective, single center study. Blood Coagulation and Fibrinolysis 2013, 24: 862-868. [ Links ]
37. Mendarte L, Munne M, et al. Use of Human Prothrombin Complex Concentrate in patients with Acquired Deficiency an Active or in High-Risk Severe Bleeding. Journal of Coagulation Disorders 2010. [ Links ]
38. AEMPS, Ficha técnica Beriplex®, consultada el 23 de Agosto de 2015. http://www.aemps.gob.es/cima/pdfs/es/ft/69890/FT_69890.pdf. [ Links ]
39. Siddiq F, Jalil A, et al. Effectiveness of factor IX complex concentrate in reversing warfarin associated coagulopathy for intracerebral hemorrhage. Neurocrit Care 2008;8:36-41. [ Links ]
40. Huttner HB, Schellinger PD, et al. Hematoma growth and outcome in treated neurocritical care patients with intracraneal hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006;37:1465-70. [ Links ]
41. Herm Jan M Brinkman. Global assays and the management of oral anticoagulation. Thrombosis Journal (2015) 13:9. [ Links ]
42. Babilonia K. and Trujillo T. The role of prothrombin complex concentrates in reversal of target specific anticoagulants. Thrombosis Journal 2014, 12:8. [ Links ]
43. AEMPS, Ficha técnica Pradaxa®, consultada el 23 de Agosto de 2015. http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_Product_Information/human/000829/WC500041059.pdf. [ Links ]
44. Stanworth SJ, Brunskill SJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol 2004,126:139-152. [ Links ]
45. Kozek-Langenecker, et al. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Critical Care 2011, 15:R239. [ Links ]
46. Schimp, et al. Impact of fibrinogen concentrate alone or with prothrombin complex concentrate (+/- fresh frozen plasma) on plasma fibrinogen level and fibrin-based clot strength (FIBTEM) in major trauma: a retrospective study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2013, 21:74. [ Links ]
47. Peyrou V, Lormeau JC, Herault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood Thromb Haemost 1999, 81:400-406. [ Links ]
48. CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32. [ Links ]
49. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg 147:113-9. [ Links ]
50. Yank V, Tuohy CV, Logan AC, Bravata DM, Staudenmayer K, Eisenhut R, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor Vila for off-label indications. Ann Intern Med. 2011;154:529-40. [ Links ]
51. Yunos NM, Bellomo R, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012; 308(15):1566-72. [ Links ]
52. Perner, A., et al. Hydroxyethylstarch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012; 367(2):124-134. [ Links ]
53. Brunkhorst, F.M., et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis . N Engl J Med 2008; 358(2):125-139. [ Links ]
54. Myburgh, J.A., et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367(20):1901-1911. [ Links ]
55. AEMPS, October 2013: http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2013/NIMUH_FV_29-hidroxietil-almidon.htm. [ Links ]
56. A. Farriols Danés, et al. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sanguinis (2008) 94:221-226. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: c.gonzalez@vhebron.net
(Celia González Guerrero).
Recibido: el 1 de marzo de 2015;
aceptado: el 26 de septiembre de 2015.











 texto en
texto en