Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Farmacia Hospitalaria
versão On-line ISSN 2171-8695versão impressa ISSN 1130-6343
Farm Hosp. vol.40 no.2 Toledo Mar./Abr. 2016
https://dx.doi.org/10.7399/fh.2016.40.2.9994
Use of closed systems in the Hospital Pharmacy
Uso de los sistemas cerrados en el Servicio de Farmacia
María Forte Pérez-Minayo1, Eva Castillo Bazán2, Marta Hernández Segurado2, María Ángeles Arias Moya2, Paloma Pelegrín Torres1 and Francisco Javier Bécares Martínez3
Pharmacy Unit. Hospital Universitario Fundación Jiménez Díaz.
1Pharmacy Degree. Resident Hospital Pharmacist.
2Pharmacy Degree. Hospital Pharmacy Specialist. Specialist Pharmacist in the Area of Cytostatics and Intravenous Compounds.
3Degree in Pharmacy and Medicine. Specialist in Hospital Pharmacy. Head of Department.
ABSTRACT
Objective: In the setting of the increasing use of closed systems for reconstitution and preparation of these drugs, we intend to analyze the correct use of these systems in the Hospital Pharmacy, with the objective to minimize the risks of exposure not only for those professionals directly involved, but also for all the staff in the unit, taking also into account efficiency criteria.
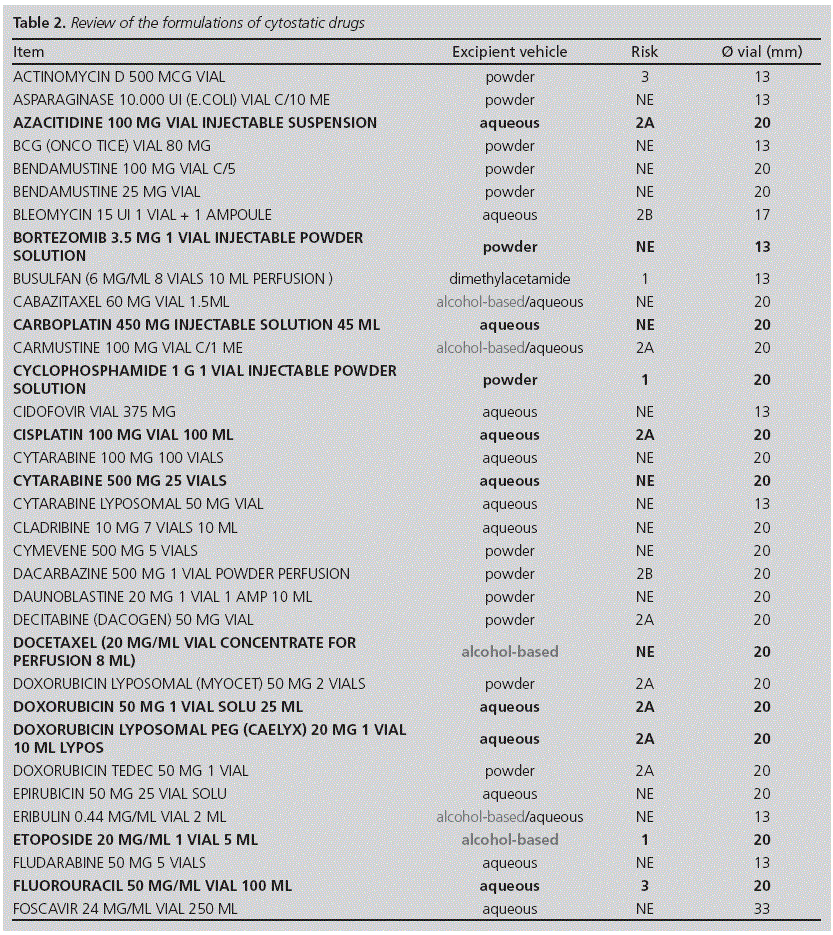
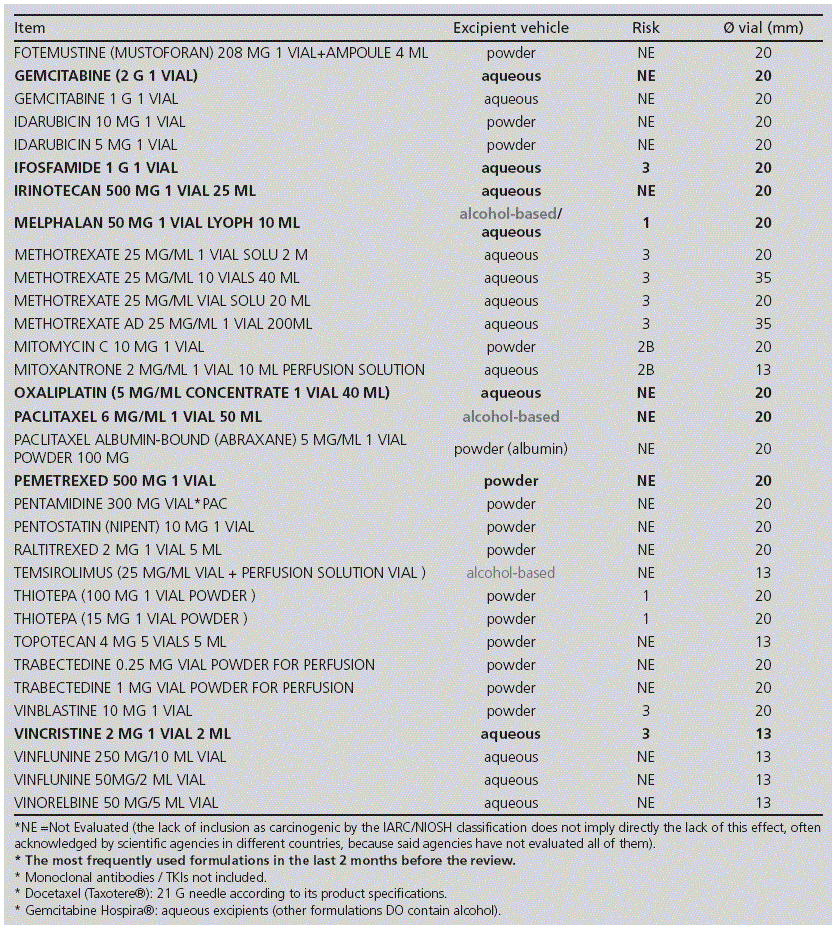
Method: Since some systems protect against aerosol formation but not from vapours, we decided to review which cytostatics should be prepared using an awl with an air inlet valve, in order to implement a new working procedure. We reviewed the formulations available in our hospital, with the following criteria: method of administration, excipients, and potential hazard for the staff handling them. We measured the diameters of the vials. We selected drugs with Level 1 Risk and also those including alcohol-based excipients, which could generate vapours.
Outcomes: Out of the 66 reviewed formulations, we concluded that 11 drugs should be reconstituted with this type of awl: busulfan, cabazitaxel, carmustine, cyclophosphamide, eribulin, etoposide, fotemustine, melphalan, paclitaxel, temsirolimus and thiotepa; these represented an 18% of the total volume of formulations.
Conclusions: The selection of healthcare products must be done at the Hospital Pharmacy, because the use of a system with an air valve inlet only for those drugs selected led to an outcome of savings and a more efficient use of materials. In our experience, we confirmed that the use of the needle could only be avoided when the awl could adapt to the different formulations of cytostatics, and this is only possible when different types of awls are available. Besides, connections were only really closed when a single awl was used for each vial. The change in working methodology when handling these drugs, as a result of this study, will allow us to start different studies about environmental contamination as a future line of work.
Key words: Cytostatic agents; Closed systems; Occupational exposure.
RESUMEN
Objetivo: En el contexto del auge de los sistemas cerrados para la reconstitución y preparación de estos fármacos, se propone analizar el uso correcto de estos sistemas en el servicio de farmacia, con el objetivo de minimizar los riesgos de exposición no solo de los trabajadores expuestos, sino de todos los trabajadores del servicio, atendiendo también a criterios de eficiencia.
Método: Puesto que algunos sistemas protegen frente a la formación de aerosoles pero no frente a vapores, decidimos revisar qué citostáticos debían prepararse con un punzón que constase de una válvula de admisión de aire para implementar un nuevo procedimiento de trabajo. Se revisaron las presentaciones disponibles en nuestro hospital atendiendo a: vía de administración, excipientes y riesgo para el personal manipulador, y se midieron los diámetros de los viales. Se seleccionaron tanto los fármacos de riesgo 1 como aquellos cuyos excipientes incluyesen vehículos alcohólicos, susceptibles de formar vapores.
Resultados: De las 66 presentaciones revisadas, un total de 11 fármacos debían reconstituirse con este tipo de punzón: busulfán, cabazitaxel, carmustina, ciclofosfamida, eribulina, etopósido, fotemustina, melfalán, paclitaxel, temsirolimús y tiotepa; representando un 18% respecto al volumen total de presentaciones.
Conclusiones: La selección de los productos sanitarios debe realizarse desde los servicios de farmacia, ya que la utilización de un sistema con válvula de admisión de aire para tan solo los fármacos seleccionados supuso un ahorro y un empleo más eficiente del material. Desde nuestra experiencia comprobamos que el uso de la aguja solo podía relegarse si el punzón se adaptaba a las diferentes presentaciones de citostáticos, y esto solo se consigue disponiendo de varios tipos de punzones. Además, las conexiones solo estaban realmente cerradas si se utilizaba un punzón por cada vial. Con el cambio en la metodología de trabajo a la hora de manipular estos fármacos, producida como resultado de este estudio, se pretenden realizar estudios de contaminación ambiental en una línea de trabajo futuro.
Palabras clave: Agentes citostáticos; Sistemas cerrados; Exposición ocupacional.
Contribution to scientific literature
To minimize the risks represented by handling cytostatic agents, not only for the staff involved but also for all professionals working in the Hospital Pharmacy.
There are certain publications within scientific literature addressing the administration of these drugs with closed systems, mostly targeted at nursing staff in Day Hospitals; but there are very limited data about how to handle these drugs in the Hospital Pharmacy taking into account their characteristics. Given the increasing concerns about the potential occupational risk involved in handling these drugs, we believe it is important to publish this type of procedures, in order to create awareness in other hospitals. The outcomes reached will involve a change in working methodology at the time of drug reconstitution.
Introduction
The term cytostatic includes a wide group of medications with very different mechanisms of action, but with the common characteristic of interrupting the cellular cycle at any of its stages. This property leads to their use in the treatment of neoplastic diseases, as single therapy or in combination with radiotherapy and/or surgery.
The constant evolution of hospital protocols, the use of new techniques, and the launch of new medications, have allowed an increase in the number of treatable patients and success expectations. Despite this, it must not be forgotten that these are very active drugs, with a high potential toxicity. There are data showing that a continuous and prolonged exposure to small doses can have mutagenic and carcinogenic effects on the staff handling them.
Given that it has not been possible to determine clearly the toxic effects at long term of the exposure to these drugs (mostly due to the inconsistencies between the different tests used for determining toxicity), the potential occupational risk entailed and the consequences they can cause, it becomes essential to adopt measures to help to reduce said exposure, and to guarantee optimal working conditions. In this sense, the most adequate action will be prevention.
In order to prevent any possible harmful effects caused by inadequate handling, an appropriate work system must be implemented, and certain action measures must be adopted when faced with any situation which involves cytostatic medications.
Moreover, the centralization of these procedures in Hospital Pharmacy Units will guarantee higher safety for the worker and for the environment, reducing as much as possible the risk of exposure1-4.
One of the most usual sources of environmental contamination by cytostatics, both during reconstitution and in administration, is the use of standard syringes which generate aerosol at the moment of being extracted of the container through the septum. There is also aerosol when the product contained in the inner walls of the syringe is expelled out during plunger withdrawal. In order to avoid this type of contamination, it is recommended to use the so-called Closed Systems5.
After being launched in the market, Closed Systems have been gradually implemented in hospitals with the aim of eliminating needles. A Closed System Drug Transfer Device refers to a system which does not allow the transfer of environmental contaminants mechanically inside the device, or the leak of dangerous molecules outside of it. In principle, a closed system is a device which prevents the exchange of non-filtered air or contaminants with the ambient air6.
In Spain, these devices are considered healthcare products, regulated by Royal Decree 1591/2009, and classified within the Class IIa7. On the other hand, occupational exposure to cytostatic drugs is included within the setting of rules about protection of workers against risk related with exposure to carcinogenic agents (Royal Decree 665/97); and therefore, according to its ruling, the use of Closed Systems should be prioritized8.
We cannot forget that it is the role of the Pharmacist to select those healthcare products to guarantee environmental safety, microbiological safety, convenience, optimal cost, and reduction in workload.
Since approximately one year and a half ago, our hospital has been incorporating closed systems, both for preparation and administration of cytostatic agents.
This was started by the Day Hospital, where the volume of treatments reaches approximately 1000 administrations per month, and there are 6 workers, including nurses and nursing assistants.
Subsequently, the implementation in Day Hospital was followed by the rest of the hospital units involved. During 7 days, training was conducted at the Hospital Pharmacy Unit with those nurses and pharmacy technicians who conduct work in the laboratory fume hood and with a workload close to 2000 preparations per month; afterwards, training continued with the Hospitalization Units for Oncology and Haematology.
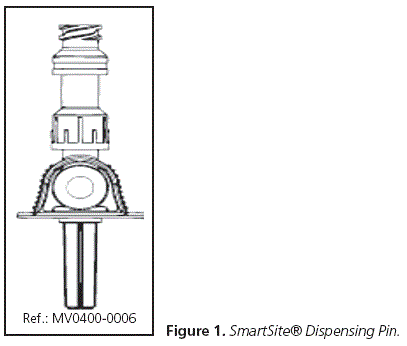
On our hand, in the Hospital Pharmacy and for the area relevant to us, which is preparation, a universal awl was initially chosen as the device use for reconstitution of these drugs. The model selected (SmartSite® Dispensing Pin) (Figure 1) could be connected to all vials.
Awls are perforators for vial access without a needle for reconstitution and dilution of medications. The FDA (Food and Drug Administration) requires all devices to meet the following criteria: being airtight, non-drip, and preventing microbiological contamination (0.22 micron filter)9.
After approximately one year and a half of usage, it was observed that the implementation of this system had not led to the elimination of the use of needles by the staff in charge of preparation; and therefore, there was no elimination of the risks entailed. It was also observed that the universal awl was not used in a high proportion of the preparations (only in approximately 40%), due to different reasons: awl quality, poor connection of the awl to the different vial sizes, worse utilization of small rests of drugs, etc. One single awl per vial was not used either, and therefore connections continued being non-closed. Besides, the device available protected the working staff against aerosols, but not against the vapours generated by some drugs (in the same way as HEPA filters, which are able to trap particles and aerosols, but not vapours, which would therefore move around freely).
The resources available so far in the Hospital Pharmacy for cytostatic preparation were: extensions, 3-part luer-lock syringes, and universal awls (Figure 1) which presented the following characteristics:
• Drug-transfer awl (with ventilation)
• Needleless system
• 0.1ml purge volume
• System length: 6.4cm
• Universal connector
• Hydrophobic filter for air entry: it prevents an increase in pressure in the vial.
The objective of this article is to review the suitability of the Closed Systems used at the Hospital Pharmacy for reconstitution of the cytostatic drugs used in our centre, which is necessary in order to ensure the safety and efficiency of the process.
Secondarily, it was considered to quantify the savings represented by using these materials according to the review conducted.
Method
A working procedure was implemented within the setting of the compulsory periodical review of all Standard Working Procedures (SWPs) for the Hospital Pharmacy, and given the concerns about an increasingly higher occupational risk, taking a closer look at the adequate use of closed systems during the reconstitution process for cytostatic drugs.
After the bibliographic review, it was recommended to have different types of awls available, in order to adapt to the characteristics of the different cytostatic medications, so that the use of needles was no longer necessary, and that all the staff, both handling and not handling these drugs, became protected against those drugs which generated vapours. This would achieve a complete implementation of the use of closed systems, without representing a high increase in the cost of this type of healthcare materials; that is to say, each type of awl would be used according to the characteristics of the medication.
The primary variable of the study was the use of the most adequate awl according to the characteristics of the drug.
A review of the product specifications was conducted for the different awls selected, as well as for the different cytostatic drugs, in order to be able to reconstitute the vials using different types of awls based on the potential level of contamination of the compound, and the level of carcinogenicity of the cytotoxic drug.
The variables considered were:
- The most frequently used drugs (in number of vials), according to the use within the two last months; the study was subsequently extended in order to include all the formulations available in the hospital. Monoclonal antibodies were initially excluded, as these are not Hazardous Drugs (drugs which require additional handling and entail risk of exposure for healthcare professionals and those who can get in contact inadvertently with them10) or chemotherapy in its strict sense.
- Perishable / way of administration: Preparation in syringe or saline solution.
- Vehicles of the drug: Review through product specifications of the excipients of all our formulations for cytostatic drugs11, detecting those with alcohol-based solvents (those likely to generate vapours).
- Risk for the staff handling the different cytostatic drugs: According to the classification by the IARC (International Agency for Research on Cancer)12, based on tests on carcinogenesis, and the classification by the NIOSH (National Institute for Occupational Safety and Health)13, screening those drugs with the highest risk for the staff handling them.
- Diameter of the vial head (in millimetres): All formulations were measured.
In order to select which drugs should be reconstituted with an awl with air inlet valve (able to trap vapours), both the carcinogenic potential and the excipients of the drugs were assessed. In order to evaluate the level of carcinogenicity, the classification by the IARC12 and the classification by the NIOSH13 were used, and those drugs with the highest risk were selected (Risk 1).
The selection criteria for the most adequate awl were:
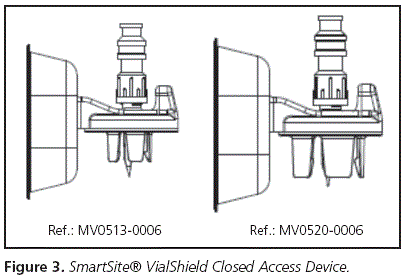
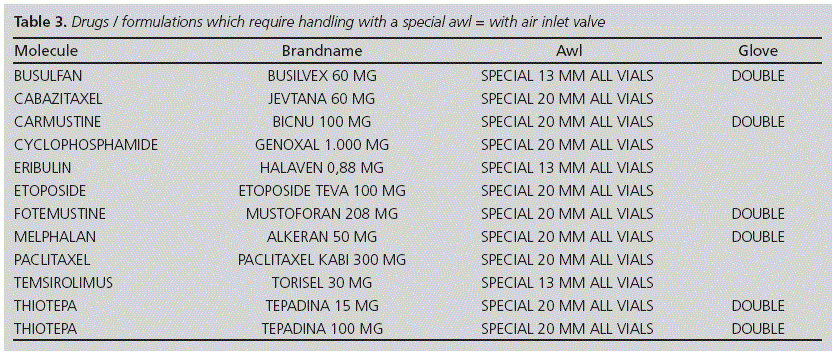
a. Awl with air inlet valve: Cytostatic drugs with Risk 1 for the staff, as well as those with excipients containing alcohol-based vehicles, should be reconstituted using the awl with air inlet valve Figure 3), measuring 13 or 20 mm depending on the head of the vial (one per each vial); some of the drugs met both criteria.
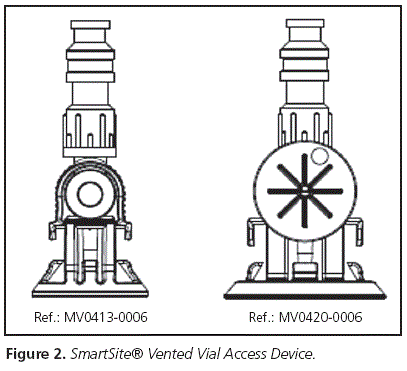
b. Basic awl: In principle, the other awl can be used for the rest of the drugs, given their characteristics (measuring 13 or 20 mm depending on the head of the vial, and also one per each vial) (Figure 2).
After this, the estimated costs of the introduction of the awl with air inlet valve were assessed. Two scenarios were considered: one where only this type of awl would be used in all cases, and another where it would only be used for those drugs which require it. The estimate was prepared taking into account the use within the 2 last months before the review, and considering that the cost of the awl with air inlet valve is approximately 4 times higher than the cost of the normal awl.
Data collection was conducted through an Excel spreadsheet. The sample size was not calculated, because the study was conducted based on the formulations available in our centre. The objective was descriptive; and therefore, a descriptive analysis was conducted for the variables previously described. The study variables were qualitative, and were expressed as counts or percentages.
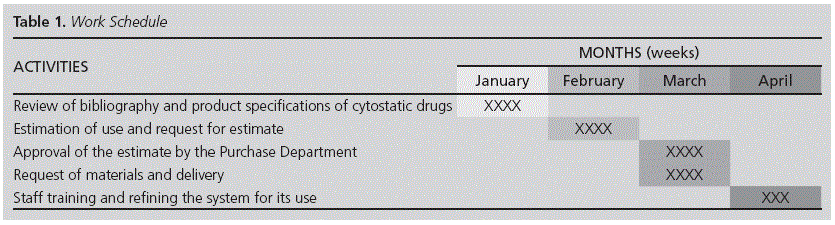
The work schedule appears on Table 1.
Results
Table 2 shows the results of the study on the formulations of cytostatic drugs available in our centre. Out of the 66 formulations assessed, there were 11 drugs in our hospital which turned out to be likely to require special handling: busulfan, cabazitaxel, carmustine, cyclophosphamide, eribulin, etoposide, fotemustine, melphalan, paclitaxel, temsirolimus, and thiotepa (15 mg and 100 mg) (see Table 3), which represented 18% (12/66) of the total volume of formulations.
Regarding the measurements of the vial diameters, practically all of them were 20mm or 13mm.
However, it was considered necessary to have two types of awl available, each one in two sizes:
- A simpler one, and similar to the one available so far (but better adapted to each vial) (Figure 2), with the following characteristics:
• Device for access to transfer
• Needleless system
• Purge volume: 0.1 ml
• System length: 6 cm
• Vented filter
• Aerosol trap
• Diameter of the vial access device: 13 and 20 mm;
- and another one, more sophisticated and with air inlet valve, for those drugs which required protection against vapours (Figure 3); the following are its most important characteristics:
• Device for access to transfer
• Needleless system
• Purge volume: 0.1 ml
• System length: 6.5cm
• Air inlet valve
• Diameter of the vial access device: also 13 and 20 mm.
The estimated annual use of awls with air inlet valve (Figure 3) was:
• 13 mm SmartSite® VialShield Closed Vial Access Device awls " approximately 300/year
• 20 mm SmartSite® VialShield Closed Vial Access Device awls " approximately 400/year
Regarding cost, an estimate was prepared with the foreseen annual use of awls. We considered two scenarios: an overall one, assuming that all drugs presented the same risk and were likely to generate vapours, and a specific one, taking into account the study conducted. In the first one, using the awl with air inlet valve for all the formulations (around 24,000 total preparations / year, extrapolating the data for monthly preparations obtained from our application Farmis Oncofarm® at 12 months), the annual spending on awls was estimated in 105,600 €/year. In the second one, if the awl with air inlet valve was used only for the formulations selected (around 4,300 preparations/year), and the basic awl for the rest of preparations (estimated by difference), the estimated annual spending was reduced to 42,560 €/ year. Therefore, the use of the awl with air inlet valve according to Scenario 2 represented annual savings of approximately 60% compared to its use according to Scenario 1, thus achieving a more efficient use of awls.
Discussion
Concerns about a potential occupational risk appeared after the publication by Falck in 1979, which showed evidence, through the application of the Ames Test, of the presence of mutagenicity in urine concentrates of nurses who handled cytostatic drugs. The values of mutagenicity obtained were higher than those of the staff not exposed, who were used as control, and increased as the week progressed, thus suggesting that the source of mutagenicity could be the absorption of cytostatics as a consequence of occupational exposure14.
At the beginning of the eighties, The Occupational Safety and Health Administration (OSHA) started to become concerned about the occupational exposure of healthcare staff to these substances, and in 1999 they published a technical manual about the control of occupational exposure to cytotoxic compounds15. More recently, The National Institute for Occupational Safety and Health (NIOSH) and The American Society of Hospital Pharmacists (ASHP) have published recommendations for safe handling of cytotoxic drugs16,17.
On the other hand, there are various published studies which intend to quantify the level of exposure of healthcare staff, with the objective of determining a threshold level, and which state the existence of contamination in the work surfaces during the handling of cytotoxic compounds18,19.
Regarding the need for special handling of those compounds which contain alcohol-based excipients likely to generate vapours, and which therefore represented real contamination, Connor and col. concluded that some cytostatic drugs presented a pressure of vapour low enough to cause vaporization at room temperature; this study demonstrated that cyclophosphamide, carmustine and mechlorethamine behaved like mutagens in Ames test, if the cytostatic and the reactive (the culture plates) were put together in a closed setting at a temperature of 23oC, without needing to be in direct contact (adding on the cytostatic to the culture plates)20. More recently, NIOSH suggested a protocol to determine the efficacy of closed systems in vapour containment, using isopropyl alcohol as tracer21.
Even if we have not yet conducted surface contamination tests in our centre after implementing these systems, there are various studies supporting the use of closed systems22,23.
In spite of current recommendations already establishing drug-transfer devices as measures for occupational protection and prevention, that is to say, closed and needleless systems (NIOSH), because they represent the only way to guarantee both the asepsis of the compounds and the protection of the handling staff24, there are very limited data about the use of these systems in Hospital Pharmacies according to drug characteristics.
Our working procedure has been recently introduced, and has been in place for some months only, but it has represented a new approach to work in the area of cytostatic drugs. The workers have adapted well, and are actively involved so that the improvement practice will really represent a benefit in their working conditions. Besides, using the awl with air inlet valve for a group of medications only, instead of for all the preparations, entails savings in healthcare materials.
In the case of monoclonal antibodies, these are potentially toxic drugs too, but not cytostatic as such, because they present a different toxicity profile where normal cells are not damaged. Therefore, there is lower danger in their handling. In this case, the main concern was guaranteeing the stability of the open vials. Data available for both awls show that, by featuring a 0.22 micron filter which traps bacteria, microbiological stability is ensured during:
• For the SmartSite® VialShield Closed Vial Access Device awl (Figure 3) » 7 days
• For the SmartSite® Vented Vial Access Device awl (Figure 2) » 96 hours
With all this, we intend to apply a criterion of efficiency, and finally decided to use the awl with air inlet valve for the last of the vials in each treatment, in case it was a monoclonal antibody (and this way, being able to keep small rests of drug in case it became necessary to make the most of vials), and to continue using blunt needles for the rest of vials, because if we decided to use an awl per vial in the case of monoclonal antibodies, the cost was increased to a great extent. Besides, the awl is not able to extract the whole volume of the antibody, and this would lead to small losses of product, which would ultimately entail a high annual cost.
After refining this new methodology of work for its use, we have finally implemented it on April, 2015, using the awl with air inlet valve only for those selected formulations; therefore, from now on we will consider if we need to make some modification in the work method based on daily practice.
In terms of the limitations of our review, some formulations did not have the standard sizes of the awls included (13 or 20 mm), so we continue having a small stock available of the awl with universal connector for their handling. Our review was been conducted with those formulations available in our hospital, but not with all formulations available in the market. It is worth noting that there are various devices currently available in the market, so the information provided by their different manufacturers should be reviewed in order to check the characteristics of all systems.
In a future line of work, tests for contamination in the environment and work surfaces could be conducted, to assess whether in our centre, and according to what is stated in scientific literature, a reduction in contamination has also been achieved after the implementation of these systems.
Conflict of interests
We the authors declare the lack of existence of any type of conflicts of interests.
Bibliography
1. Rey M., Corrales E., Serra MA., Clopés A. Manipulación y administración de citostáticos (Monografía en Internet). Madrid: Instituto Catalán de Oncología; 2006. (n.d). Disponible en: http://www.combino-pharm.es/wp-content/uploads/2014/07/MONOGRAFIA_CITOSTATICOS.pdf [ Links ]
2. Autoría múltiple. Guía de Buenas Prácticas para trabajadores profesionalmente expuestos a agentes citostáticos. (Monografía en Internet). Madrid: Escuela Nacional de Medicina del Trabajo. Instituto de Salud Carlos III. Ministerio de Economía y Competitividad. Martínez de Aramayona López MJ, Sánchez-Uriz MA. Coordinadoras; 2014 (22/03/2014). Disponible en: http://gesdoc.isciii.es/gesdoccontroller?action=download&id=26/03/2014-199edf956b
[ Links ]3. Association paritaire pour la santé et la sécurité du travail du secteur affaires sociales (ASSTSAS). Safe Handling of Hazardous Drugs. Prevention Guide (Monografía en Internet). Montreal; 2008. (n.d). Disponible en: https://www.irsst.qc.ca/media/documents/PubIRSST/CG-002.pdf [ Links ]
4. Connor T, McLauchlan R, Vandenbroucke J. ISOPP Standards of Practice. Safe Handling of Cytotoxics. J Oncol Pharm Practice Supp. 2007; 13: 1-81. [ Links ]
5. Guardino Solá X. Exposición laboral a compuestos citostáticos: sistemas seguros para su preparación. Instituto Nacional de Seguridad e Higiene en el Trabajo (INSHT); 2015. NTP 1.051. (05/10/2015). Disponible en: http://www.insht.es/InshtWeb/Contenidos/Documentacion/NTP/NTP/Ficheros/1043a1054/n tp-1051w.pdf [ Links ]
6. National Institute for Occupational Safety and Health (NIOSH). Definition of Closed-System Drug-Transfer Devices. Ann Occup Hyg. 2009; 53(5): 549. [ Links ]
7. Real Decreto 1591/2009 por el que se regulan los productos sanitarios. Boletín Oficial del Estado 268 de 6 de noviembre de 2009. [ Links ]
8. Real Decreto 665/97 sobre la protección de los trabajadores contra los riesgos relacionados con la exposición a agentes cancerígenos durante el trabajo. Boletín Oficial del Estado 124 de 24 de mayo de 1997. [ Links ]
9. Elaboración de productos estériles: Hazardous Drugs. (Monografía en Internet). Curso Precongreso para Formadores de Manipuladores en Área Estéril. 59 Congreso Sociedad Española de Farmacia Hospitalaria. Valladolid: Sociedad Española de Farmacia Hospitalaria; 2014 (n.d). Disponible en: http://gruposdetrabajo.s efh.es/nutricion/images/stories/documentos/documentos/CursoPrecongreso2014/ElaboracionProductosEsterilesHazardousDrugsCP2014.pdf [ Links ]
10. Clark C. Hazardous drug exposure-preparation to administration. Hosp Pharm Eur. 2015; 77. [ Links ]
11. Agencia Española del Medicamento y Productos Sanitarios. (n.d). Disponibles en http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=detalleForm [ Links ]
12. Agencia Internacional para la Investigación del Cáncer (IARC). Agents Classified by the IARC Monographs. (Monografías en Internet). Volumes 1-113. (24/08/2015). Disponible en: http://monographs.iarc.fr/ENG/Classification/latest_classif.php [ Links ]
13. National Institute for Occupational Safety and Health (NIOSH). List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings. (Monografía en Internet); 2014. 2014-138. (15/05/2015). Disponible en: http://www.cdc.gov/niosh/docs/2014-138/pdfs/2014-138_v3.pdf [ Links ]
14. Protocolo Manejo Seguro de Citostáticos. (Monografía en Internet). Servicio Riojano de Salud; 2012. (n.d). Disponible en: https://www.riojasalud.es/rrhh-files/rrhh/protocolo-manejo-seguro-de-citostaticos-2999 .pdf [ Links ]
15. Occupational Safety and Health Administration (OSHA). OSHA Technical Manual. (02/11/2014). Disponible en: https://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html [ Links ]
16. USA NIOSH ALERT. Preventing Occupational Exposure to Antineoplastic and other Hazardous Drugs in Health Care Settings. NIOSH; 2004. (05/09/2014). Disponible en: http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf [ Links ]
17. American Society of Health-System Pharmacists (ASHP). Guidelines on preventing medication errors with antineoplastic agents. Am J. Health-Syst Pharm. 2002; 59: 1648-68. [ Links ]
18. González Álvarez A, López-Montenegro Soria MA, Albert Marí A, Martínez Gómez MA, Porta Oltra B, Jiménez Torres NV. Exposición a fármacos citotóxicos en el personal sanitario. Farm Hosp. 2012; 36 (5): 368-373. [ Links ]
19. Fleury-Souverain et al. Evaluation of chemical contamination of surfaces during the preparation of chemotherapies in 24 hospital pharmacies. Eur J Hosp Pharm. 2015; 22 (6): 333-341. [ Links ]
20. Cajaraville G., Tamés MJ. Guía de manejo de medicamentos citostáticos. (Monografía en Internet). Instituto oncológico. San Sebastian. (n.d). Disponible en: http://www.sefh.es/bibliotecavirtual/citostaticos/guiamanejocitos.pdf [ Links ]
21. National Institute for Occupational Safety and Health (NIOSH). A vapor containment performance protocol for closed system transfer devices using during pharmacy compounding and administration of hazardous drugs. Centers for Disease Control and Prevention; 2015. CDC-2015-0075. (08/09/2015). Disponible borrador en: http://www.regulations.gov/#!documentDetail;D=CDC-2015-0075-0003 [ Links ]
22. Sessink PJ, Connor TH, Jorgenson JA, Tyler TG. Reduction in surface Contamination with antineoplastic drugs in 22 hospital pharmacies in the US following implementation of a closed-system drug transfer device. J Oncol Pharm Pract. 2011; 17(1): 39-48. [ Links ]
23. Yoshida J, Tei G, Mochizuki C, Masu Y, Koda S, Kumagai S. Use of a closed system device to reduce occupational contamination and exposure to antineoplastic drugs in the hospital work environment. Ann Occup Hyg. 2009; 53(2): 153-160. [ Links ]
24. Uribe Llopis P. Manejo de citostáticos, medidas de prevención y vigilancia de la salud. II Jornada Técnica Productos Sanitarios y Medio Ambiente. Madrid; 2013 (n.d). Disponible en: http://www.madrid.org/cs/Satellite?blobcol=urldata&blobheader=application%2Fpdf&blobkey=id&blobtable=MungoBlobs&blobwhere=1352814382035&ssbinary=true [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: maria.forte@fjd.es
(María Forte Pérez-Minayo).
Recibido el 5 de octubre de 2015;
aceptado el 22 de enero de 2016.











 texto em
texto em