My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Farmacia Hospitalaria
On-line version ISSN 2171-8695Print version ISSN 1130-6343
Farm Hosp. vol.40 n.4 Toledo Jul./Aug. 2016
https://dx.doi.org/10.7399/fh.2016.40.4.10453
ORIGINALES
Persistence to single-tablet regimen versus less-drug regimen in treatment experienced HIV-infected patients on antiretroviral therapy
Persistencia entre las estrategias terapéuticas single-tablet y less drug en pacientes VIH+ previamente tratados
Rocío Jiménez-Galán1, María-Rosa Cantudo Cuenca1, María Aguas Robustillo-Cortés1, Y. Borrego Izquierdo1, Carmen Victoria Almeida-Gonzalez2 and Ramón Morillo-Verdugo1
1Department of pharmacy. Valme university Hospital, Seville.
2Research Unit. Valme university Hospital, Seville. Spain.
ABSTRACT
Background: Decreased antiretroviral therapy persistence is associated with increased rates of virologic failure, development of antiretroviral resistance, and increased morbidity and mortality. Different therapeutic strategies, such as single-tablet regimens (STR) and less-drug regimens (LDR), have been developed in order to simplify antiretroviral therapy (ART) and increase persistence.
Objectives: The primary objective was to compare antiretroviral persistence among patients receiving STRs and patients receiving LDRs. A secondary objective was to identify factors associated with non-persistence.
Methods: This was a retrospective study that included treatment-experienced HIV-infected patients who received ART based on STR or LDR. Baseline patient characteristics collected included demographic information, HIV risk transmission, substance abuse during the therapy, presence of psychiatric disorder and hepatitis B or C virus infection. Kaplan-Meier analysis and Log rank was utilized to compare persistence to STR and LDR. To identify independent predictors of non-persistence we developed a multivariate Cox regression analysis.
Results: A total of 244 patients were included, 176 with STR and 68 with LDR. 60 (34.1%) patients discontinued in the STR group and 13 (19.1%) in the LDR group. The Cox regression model showed that the only variable associated with higher risk of non-persistence was the substance abuse (HR = 2.59; p = 0.005). Adverse events were the main reason for ART discontinuation in the STR group and virologic failure in the LDR group.
Conclusions: Persistence to STR and LDR seems to be similar in pretreated HIV-infected patients. Drug abuse was the only factor identified with a higher risk of non-persistence.
Key words: HIV; Medication persistence; Antiretroviral therapy.
RESUMEN
Objetivos: Analizar y comparar la persistencia entre las estrategias basadas en Single-Tablet Régimen (STR) y Less Drug Régimen (LDR) en pacientes VIH+. El objetivo secundario del estudio fue determinar factores predictores de persistencia.
Material y métodos: Estudio observacional retrospectivo que incluyó los siguientes criterios: pacientes VIH+ con tratamiento antirretroviral (TAR) con un régimen basado en STR o LDR. Se recogieron variables demográficas, factores de riesgo de adquisición, consumo de drogas, presencia de algún trastorno psiquiátrico y coinfección por el virus de la hepatitis B o C. Para comparar la persistencia entre ambas estrategias se realizó un análisis de supervivencia de Kaplan-Meir y se aplicó el método de log-rank. Se realizó un análisis de regresión de Cox para identificar los factores predictores de persistencia.
Resultados: Se incluyeron 244 pacientes, 176 con STR y 68 con LDR. El 34,1% (n = 60) de los pacientes que recibieron un régimen STR abandonaron y en el LDR el 19,1% (n = 13). Los efectos adversos fueron la principal causa de abandono del tratamiento en los pacientes que recibieron STR y el fallo virológico en el régimen LDR. La persistencia de las estrategias STR y LDR fue similar, no encontrándose diferencias estadísticamente significativas entre ambas. El consumo de drogas fue el único factor predictivo asociado con una menor persistencia (HR = 2,59; p = 0,005).
Conclusiones: La persistencia entre los regímenes STR y LDR fue similar, no detectándose diferencias significativas entre ambos. El consumo de drogas fue el único factor independiente asociado con una menor persistencia del tratamiento antirretroviral.
Palabras clave: VIH; Persistencia de la medicación; Tratamiento antirretroviral.
Contribution to the current scientific evidence
This study shows a finding that has not been previously studied in treatment-experienced HIV-infected patients. We find that persistence to single-tablet regimen and less-drug regimen is similar among treatment experienced HIV-infected patients. Decreased persistence for HIV treatment is associated with higher rates of virologic failure, development of antiretroviral resistance, and increased morbidity and mortality. Different factors have been associated with lower persistence to antiretroviral treatment (ART), among them, regimen complexity. In recent years, the emergence of single-tablet regimen (STR) and less-drug regimen (LDR) have let reduce pill burden and improve quality of life. To date, persistence to these types of simplification strategies has not been compared. Also, most of studies have determined persistence to ART in naive patients. In our study, we focused in treatment experienced HIV-infected patients because LDRs have been associated with a higher frequency of blips and most of clinical guidelines recommend the use of this strategy in patients who have achieved viral suppression on an initial combination ART. Therefore, we find that persistence to these strategies is similar, in spite of the fact that LDRs have been associated with higher rates of virologic failure.
Introduction
The introduction of highly active combination antiretroviral therapy for HIV-infected individuals has greatly reduced human immunodeficiency virus (HIV)-related morbidity and mortality. The standard antiretroviral therapy (ART) for treatment-naive patients is a combination of three or four drugs: two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug: a protease inhibitor (PI) pharmacokinetically enhanced(boosted) by ritonavir, non-nucleoside reverse transcriptase inhibitor (NNRTI) or integrase strand transfer inhibitor1.These combinations have proven to be highly effective in both clinical trials and in clinical practice for treatment-naive and treatment-experienced patients. However, early ART regimens was often complicated and had high pill burdens, complex dosing schedules and numerous poor side effects effect profiles. However, in 2000, single tablet regimen (STR) became available with the emergence of abacavir, lamivudine and zidovudine (ABC/3TC/ZDV) twice2. Subsequently, other STRs have been marketed, such as efavirenz/emtricitabine/tenofovir (EFV/FTC/TDF), rilpivirine/emtricitabine/tenofovir (RPV/3TC/TDF), dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) and cobicistat/emtricitabine/tenofovir/elvitegravir (COBI/FTC/TDF/EVG).
The main objectives of therapy simplification are to reduce pill burden, improve quality of life, improve medication adherence, minimize short and long term toxicities, reduce the risk of virologic failure, preserve future treatment options and reduce the frequency of disease progression2.
Another possibility to simplify ART are NRTI-sparing regimens. Nucleoside reverse transcriptase inhibitor-based regimens have been associated with long-term toxicities, like lipodystrophy, mitochondrial toxicity, bone disorders, renal function impairment and increased cardiovascular risk3-6. Monotherapy with ritonavir-boosted PI (PI/r) can be a strategy for maintenance therapy due to their high potency and higher genetic barrier that leads to lessdrug resistance and possibility for once daily dosing. However, this type of regimen has been associated with a more frequent increases in HIV viral load compared to regimens consisting of two NRTIs plus PI/r. Therefore, clinical guidelines recommend the use of this strategy in patients who have achieved viral suppression on an initial combination ART1,7. Fortunately, the emergence of new classes of antiretroviral drugs has enabled the evaluation of potentially safer and more effective regimens. Promising antiretroviral regimens include combinations of lamivudine plus PI/r to prevent adverse events caused by NRTI. These combinations based on monotherapy or dual therapy with a PI/r have been called less-drug regimens (LDR).
Treatment regimen characteristics including efficacy, tolerability profile and regimen complexity have been associated with lower adherence and shorter persistence8-9. Adherence and persistence are often used interchangeably and can lead to incorrect conclusions. Adherence measure the extent to which a patient acts in accordance with a prescribed regimen within a given time period whereas medication persistence is defined as the "the duration of time from initiation to discontinuation of therapy". Decreased persistence for HIV treatment, or shorter duration on therapy, is associated with increased rates of virologic failure, development of antiretroviral resistance, and increased morbidity and mortality10.
Few studies have evaluated persistence to ART, and most of them have been focused on treatment-naive patients. New strategies of simplification should be analyzed in real world settings. Persistence to LDR and STR in treatment-experienced HIV-infected patients has not been compared. Therefore, the main objective of this study is to analyze and compare persistence among patients receiving LDRs or STRs. A secondary objective is to identify other factors associated with ART persistence.
Material and methods
Study population and data collection
We conducted a post-licensing, retrospective, single-center study that included HIV-infected outpatients who attended the pharmaceutical care office of a hospital pharmacy service between January 2007 and June 2014. Eligible participants had to be 18 years of age or older, treatment experienced, and receiving treatment with a STR or LDR. STRs included EFV/FTC/TDF or RPV/FTC/TDF in a fixed dose administered once-daily. LDRs consisted of monotherapy with a PI/r or dual dual therapy with a PI/r plus one other drug. Patients treated with LDR had to have HIV indetectable viral load with the previous regimen for at least the past six months.
Patients who started treatment with EFV/FTC/TDF and switched to RPV /FTC/TDF were considered persistent to STR strategy, except patients who discontinued for treatment failure. In the same way, patients who switch to another PI were considered persistent to LDR strategy, provided that the change was not due to virological failure. Baseline population characteristics collected included socio-demographic information, including date of birth and gender, HIV risk factors and substance abuse while on ART. We also collected presence of certain co-morbidities such as psychiatric disorders and hepatitis C and/or B virus infection.
All data were retrospectively obtained from medical laboratory and microbiology records, and review of the medical history of each patient. The study conformed to the tenets of the declaration of Helsinki. Informed consent was not obtained due to theretrospective design of the study.
Outcome measures
The primary end point was the persistence to treatment, defined as the time from starting of ART to treatment discontinuation or change based on prescriptions. We considered a permissible gap of more than seven days11. Patients who changed to another different strategy were considered as non-persistent. We compared persistence associated with STRs and LDRs. Reasons for regimen change or interruption were collected and were classified as adverse events (AEs), virologic failure, physician decision, patient wish and others. We also analyzed the influence of patient-level characteristics on ARTpersistence.
Statistical analysis
Descriptive analysis of baseline variables was carried out using frequency distributions or median and interquartile ranges (IQR). Differences in baseline characteristics of patients according to type of regimen were determined by univariate logistic regression. Kaplan-Meir curves were plotted according to type of regimen and baseline population characteristics. Log-rank tests were used to compare differences in ART persistence between groups. Multivariate Cox (proportional hazards) regression analysis was utilized in order to identify independent predictors of persistence to ART. Data analysis was performed using the Statistical Package for the Social Science (SPSS) version 20.0 for Windows (IBM Corp., Armonk, NY).
Results
Baseline characteristics
A total of 244 patients were included in the study. Median time of follow-up was 25 months (IQR: 10-54 months). Baseline characteristics stratified by the type of regimen are shown in table 1. The age was 48 years (IQR 44.0-52.7) in overall population, and STR and LDR group. Most of patients enrolled were older than 50 years old. The rate of HCV coinfection was high in both groups. On the contrary, a low proportion of patients had a psychiatric disorder. Drug abuse was slightly more frequent in the STR group statistically significant differences between both treatment groups according to age, gender, HCV or HBV coinfection, HIV risk transmission, drug abuse or presence of psychiatric disorder.
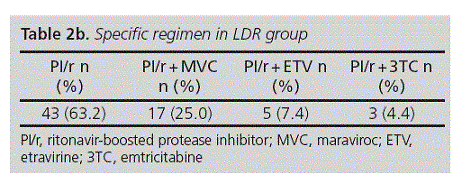
The most common STR and LDR were EFV/FTC/TDF and PI/r, respectively (Table 2a and 2b).
Persistence to ART
During the follow-up period, 60 patients (34.1%) and 13 patients (19.1%) discontinued ART treatment in the STR group and LDR group, respectively. Median time to was not reached in any of the treatment groups. The proportion of patients persistent to STR and LDR at one year of follow-up was 73% and 84%, respectively. second year 65% and 74% and 62% and 74%.
Results of Kaplan-Meier analysis stratified by type of regimen and baseline population characteristics are shown in figure 1. o statistically significant differences in ART persistence according to type of regimen, gender, presence of HCV or HBV co-infection or psychiatric disorder. On the contrary, having injection drug use as an HIV risk factor and active substance abuse while on ART were independently associated with lower persistence to ART.
Overall, the Cox regression model for this outcome (adjusted by gender, age, type of regimen, HIV risk factor, substance abuse and HCV co-infection) showed that the only patient-level variable associated withahigher risk of ART non-persistence was substance abuse during treatment with ART (Table 3).
Reasons for ART discontinuation
Reasons for ART discontinuation in patients treated with STRs and LDRs are shown in Table 4. AEs were the main reason for ART discontinuation in the STR group whereas virologic failure was the main reason for ART discontinuation in the LDR group. Another important reason for change or interruption of ART in the STR group was patient wish; this finding was not observed in the LDR group.
On the other hand, fifty four patients switched EFV/FTC/TDF to RPV /FTC/TDF. AE were the main reason (75.86% of patients) and the rest of patients were because of a physician decision.
Discussion
Results of this study show similar persistence to STR and LDRs in a real-setting cohort. Treatment characteristics have been identified as one of the key factors that contribute to persistence to ART12-15. Dosing frequency and pill burden are important treatment characteristics that impact ART persistence. Several studies have reported improved treatment persistence in HIV infected patients with regimens dosed once-daily and regimens containing fewer pills10. The emergence of STRs has been associated greater persistence compared with other regimens. In the study carried out by Taneja et al16, persistence to EFV/FTC/TDF as a fixed-dose was higher compared with other EFV-based regimens and nevirapine-based regimens. In another studies NRTI-based regimens had greater rates of persistence than PI-based regimens. However, this study also found that EFV/FTC/ TDF as a fixed-dose was the type of NRTI-based regimen associated with the longest ART persistence17.
Conversely, medication toxicity is one of the most common reasons for ART discontinuation13,18. First-line antiretroviral therapy with two NRTIs plus EFV has shown lower risk of discontinuation due totoxicity compared with two NRTIs plus ritonavir-boosted lopinavir19. The only PI that has showna similar toxicity profile toefavirenz was un boosted atazanavir20. However, when PI/r is administered in monotherapy, it hasalower toxicity profile compared with triple therapy with two NRTIs plus a NNRTI21. Results from our study are consistent with available evidence, becausetherate of discontinuation due toAE was higher in patients treated with a STR.
Another important cause of treatment discontinuation is treatment failure. LDRs and particularly monotherapy with PI/r present a greater likelihood of treatment failure7. For this reason, clinical guidelines do not recommend monotherapy with PI/r as first line antiretroviral regimen. In the same line, In our study we found a higher rate of virologic failure in the arm treated with a LDR than in those with a STR.
In our study we also determined other factors related to non-persitence to ART. Age did not impact ART persistence. These results are consistent with those obtained in three other studies17,22-23. There is only one study where younger age was associated with lower persistence24. On the other hand, while female gender has been associated with lower persistence in some studies17,25, we observed similar ART persistence between men and women in our study.
Presence of certain comorbidities has also related with lower persistence to ART in some studies, such as HCV co-infection or psychiatric diseases. Several studies have reported that HCV coinfected patients were more likely to discontinue antiretroviral mainly due to toxicity [26-28]. Increased risk of ART toxicity in HCV co-infected patients has been observed in patients with advanced HCV-related hepatic disease because most ART undergoes hepatic metabolism29. On the contrary, we did not find a relationship between HCV co-infection and persistence to ART. Differences found in our study may be due to a difference in patient characteristics, because we did not analyze severity of liver disease of the study population. Results with regard to presence of psychiatric disorder are varied9,24,30. In our study, presence of psychiatric disorders did not impact ART persistence; although future studies with larger sample sizes are needed in order to assess the influence of pyschiatric co-morbidity on ARTpersistence.
Finally, substance abuse while on ART was the only significant predictor of ART non-persistence. Our findings are consistent with results obtained in most of studies. High rates of non-persistence to ART have been reported in a study carried out among HIV-infected drug users31. Also, some studies have identified substance abuse as an important factor associated with non-persistence11,25.
Limitations
This study has several limitations. Firstly, some of limitations are due to retrospective design of the study.
AEs were the main reason for discontinuation in the STR group. This could be because most of patients included in the study were treated with EFV/FTC/TDF.
Some variables such as previous antiretroviral treatment, concomitant medications, or adherence to antiretroviral treatment were not collected in the study.
On the other hand, the study population treated with LDR was small and as a consequence, the number of patients discontinued the treatment was very low. Further studies with larger sample size are needed to prove that both strategies have similar persistence and determine clinical consequences of regimen discontinuation or change.
Conclusion
Based on our analysis persistence to STR and LDR seems to be similar in treatment-experiencedHIV-infected-patients on therapy. Drug use was the only factor identified with a higher risk of non-persistence to these types of strategies.
Bibliography
1. HIV/AIDS Guidelines-AA_Recommendations.pdf (Internet). (cited 2016 Jan 24). Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/AA_Recommendations.pdf. [ Links ]
2. Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006 Jan 19;354(3):251-60. [ Links ]
3. Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS Lond Engl. 2000 Feb 18;14(3):F25-32. [ Links ]
4. Cotter AG, Vrouenraets SME, Brady JJ, Wit FW, Fux CA, Furrer H, et al. Impact of switching from zidovudine to tenofovir disoproxil fumarate on bone mineral density and markers of bone metabolism in virologically suppressed HIV-1 infected patients; a substudy of the PREPARE study. J Clin Endocrinol Metab. 2013 Apr;98(4):1659-66. [ Links ]
5. Ryom L, Mocroft A, Kirk O, Worm SW, Kamara DA, Reiss P, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013 May 1;207(9):1359-69. [ Links ]
6. Strategies for Management of Anti-Retroviral Therapy/INSIGHT, DAD Study Groups. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS Lond Engl. 2008 Sep 12;22(14):F17-24. [ Links ]
7. gesida-guiasclinicas-2016-tar.pdf (Internet). (cited 2016 Jan 24). Available from: http://www.gesida-seimc.org/contenidos/guiasclinicas/2016/gesida-guiasclinicas-2016-tar.pdf. [ Links ]
8. Sullivan PS, Campsmith ML, Nakamura GV, Begley EB, Schulden J, Nakashima AK. Patient and regimen characteristics associated with self-reported nonadherence to antiretroviral therapy. PloS One. 2007;2(6):e552. [ Links ]
9. Willig JH, Abroms S, Westfall AO, Routman J, Adusumilli S, Varshney M, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS Lond Engl. 2008 Oct 1;22(15):1951-60. [ Links ]
10. Bae JW, Guyer W, Grimm K, Altice FL. Medication persistence in the treatment of HIV infection: a review of the literature and implications for future clinical care and research. AIDS Lond Engl. 2011 Jan 28;25(3):279-90. [ Links ]
11. Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet Lond Engl. 2010 Jul 31;376(9738):367-87. [ Links ]
12. Kiguba R, Byakika-Tusiime J, Karamagi C, Ssali F, Mugyenyi P, Katabira E. Discontinuation and modification of highly active antiretroviral therapy in HIV-infected Ugandans: prevalence and associated factors. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):218-23. [ Links ]
13. Kumarasamy N, Vallabhaneni S, Cecelia AJ, Yepthomi T, Balakrishnan P, Saghayam S, et al. Reasons for modification of generic highly active antiretroviral therapeutic regimens among patients in southern India. J Acquir Immune Defic Syndr. 2006 Jan 1;41(1):53-8. [ Links ]
14. MacArthur RD, Novak RM, Peng G, Chen L, Xiang Y, Hullsiek KH, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomised trial. Lancet Lond Engl. 2006 Dec 16;368(9553):2125-35. [ Links ]
15. Mocroft A, Youle M, Moore A, Sabin CA, Madge S, Lepri AC, et al. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. AIDS Lond Engl. 2001 Jan 26;15(2):185-94. [ Links ]
16. Taneja C, Juday T, Gertzog L, Edelsberg J, Correll T, Hebden T, et al. Adherence and persistence with non-nucleoside reverse transcriptase inhibitor-based antiretroviral regimens. Expert Opin Pharmacother. 2012 Oct;13(15):2111-8. [ Links ]
17. Juday T, Grimm K, Zoe-Powers A, Willig J, Kim E. A retrospective study of HIV antiretroviral treatment persistence in a commercially insured population in the United States. AIDS Care. 2011 Sep;23(9):1154-62. [ Links ]
18. Cicconi P, Cozzi-Lepri A, Castagna A, Trecarichi EM, Antinori A, Gatti F, et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naive patients. HIV Med. 2010 Feb;11(2):104-13. [ Links ]
19. Domingo P, Suärez-Lozano I, Torres F, Teira R, Lopez-Aldeguer J, Vidal F, et al. First-line antiretroviral therapy with efavirenz or lopinavir/ritonavir plus two nucleoside analogues: the SUSKA study, a non-randomized comparison from the VACH cohort. J Antimicrob Chemother. 2008 Jun;61(6):1348-58. [ Links ]
20. Squires K, Lazzarin A, Gatell JM, Powderly WG, Pokrovskiy V, Delfraissy J-F, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004 Aug 15;36(5):1011-9. [ Links ]
21. Arribas JR, Doroana M, Turner D, Vandekerckhove L, Streinu-Cercel A. Boosted protease inhibitor monotherapy in HIV-infected adults: outputs from a pan-European expert panel meeting. AIDS Res Ther. 2013;10(1):3. [ Links ]
22. Vo TTN, Ledergerber B, Keiser O, Hirschel B, Furrer H, Battegay M, et al. Durability and outcome of initial antiretroviral treatments received during 2000-2005 by patients in the Swiss HIV Cohort Study. J Infect Dis. 2008 Jun 15;197(12):1685-94. [ Links ]
23. Pence BW, Ostermann J, Kumar V, Whetten K, Thielman N, Mugavero MJ. The influence of psychosocial characteristics and race/ ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008 Feb 1;47(2):194-201. [ Links ]
24. Li X, Margolick JB, Conover CS, Badri S, Riddler SA, Witt MD, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005 Mar 1;38(3):320-8. [ Links ]
25. Kavasery R, Galai N, Astemborski J, Lucas GM, Celentano DD, Kirk GD, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009 Apr 1;50(4):360-6. [ Links ]
26. Mocroft A, Phillips AN, Soriano V, Rockstroh J, Blaxhult A, Katlama C, et al. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: increased incidence of stopping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Hum Retroviruses. 2005 Sep;21(9):743-52. [ Links ]
27. Mocroft A, Rockstroh J, Soriano V, Ledergerber B, Kirk O, Vinogradova E, et al. Are specific antiretrovirals associated with an increased risk of discontinuation due to toxicities or patient/physician choice in patients with hepatitis C virus coinfection? Antivir Ther. 2005;10(7):779-90. [ Links ]
28. Hooshyar D, Napravnik S, Miller WC, Eron JJ. Effect of hepatitis C coinfection on discontinuation and modification of initial HAART in primary HIV care. AIDS Lond Engl. 2006 Feb 28;20(4):575-83. [ Links ]
29. McCabe SM, Ma Q, Slish JC, Catanzaro LM, Sheth N, DiCenzo R, et al. Antiretroviral therapy: pharmacokinetic considerations in patients with renal or hepatic impairment. Clin Pharmacokinet. 2008;47(3):153-72. [ Links ]
30. Ahdieh-Grant L, Tarwater PM, Schneider MF, Anastos K, Cohen M, Khalsa A, et al. Factors and temporal trends associated with highly active antiretroviral therapy discontinuation in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2005 Apr 1;38(4):500-3. [ Links ]
31. Ing EC, Bae JW, Maru DS-R, Altice FL. Medication persistence of HIV-infected drug users on directly administered antiretroviral therapy. AIDS Behav. 2013 Jan;17(1):113-21. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: jimenezgalanrocio@gmail.com
(Rocío Jiménez-Galán).
Recibido el 15 de enero de 2016;
Aceptado el 22 de mayo de 2015.