Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Farmacia Hospitalaria
versión On-line ISSN 2171-8695versión impresa ISSN 1130-6343
Farm Hosp. vol.40 no.5 Toledo sep./oct. 2016
https://dx.doi.org/10.7399/fh.2016.40.5.10118
ORIGINALES
Clinical outcomes of the inclusion of the therapeutic drug monitoring report in the electronic clinical record
Resultados en salud de la integración del informe de monitorización farmacocinética en la historia clínica electrónica
Marina Sáez Belló1, Ana Moya Gil1, M.a Ángeles López-Montenegro Soria2, Pablo Sánchez Sancho1, Pau Frías Ruiz1 and Mónica Climente Martí1
1Pharmacy Unit. Hospital Universitario Dr. Peset. Valencia,
2Pharmacy Unit. Hospital Lluís Alcanyís de Xátiva, Valencia. Spain.
ABSTRACT
Objectives: To assess the inclusion of the Therapeutic Drug Monitoring Report (TDMR) in the Electronic Clinical Record (ECR).
Method: An observational ambispective cohort study with a duration of 149 days: PRE (retrospective, 49 days) with the TDMR printed in paper, and POST (prospective, 100 days) with the TDMR included in the ECR.
Exclusion criteria: Patients not hospitalized, applications for Therapeutic Drug Monitoring by Critical Care and Neonatal Units, as well as monitoring with an objective other than dose adjustment.
Variables: Number of TDMRs prepared, number of patients admitted with TDMR, time of delay for treatment adjustment, defined as the number of adjustments made to the treatment within over or under 24 hours from the time of TDMR preparation, and medication errors (MEs) associated with said delay, as well as the degree of acceptance of the TDMR.
Results: 690 TDMRs were conducted in 391 patients, 339 in PRE (n = 206) and 351 in POST (n = 185). The number of treatment modifications made in under 24 hours increased from 73.9% in PRE to 87.3% in POST [RR = 1.2 (CI95% = 0.97-1.43). We identified 35 patients with ME, 9.7% of them in PRE and 8.1% in POST (RR = 0.84 (CI95% = 0.44-1.58)]. The degree of acceptance of the pharmacist recommendation increased from 53.3% in PRE to 68.3% in POST [RR = 1.3 (CI95% = 1.021.62)].
Conclusions: The inclusion of the Therapeutic Drug Monitoring Report (TDMR) in the Electronic Clinical Record increases the degree of acceptance of recommendations, and may reduce the delay in treatment modifications, reducing MEs and improving the process quality in terms of efficacy and safety.
Key words: Technological development; Electronic clinical records; Health information systems; Pharmacokinetics; Medication errors; Organizational innovation.
RESUMEN
Objetivos: Valorar la integración del informe de monitorización farmacocinética (IMFC) en la historia clínica electrónica (HCE).
Método: Estudio observacional ambispectivo de cohortes de 149 días de duración: PRE (retrospectiva, 49 días) con emisión del IMFC en papel y POST (prospectiva, 100 días) con emisión del IMFC integrado en HCE.
Criterios de exclusión: Pacientes no ingresados, solicitudes de monitorización farmacocinética de unidades de críticos y neonatos, así como monitorizaciones cuyo objetivo no era el ajuste posológico.
Variables: Número de IMFC elaborados, número de pacientes ingresados con IMFC, tiempo de demora de las adecuaciones del tratamiento definidas como número de adecuaciones realizadas en el tratamiento en más o en menos 24 horas respecto al momento de emisión del IMFC, y errores de medicación (EM) asociados a dicha demora, así como grado de aceptación del IMFC.
Resultados: Se realizaron 690 IFC en 391 pacientes, 339 en PRE (n = 206) y 351 en POST (n = 185). El número de modificaciones realizadas en menos de 24 horas aumentó del 73,9% en PRE al 87,3% en POST [RR = 1,2 (IC95% = 0,97-1,43)]. Se identificaron 35 pacientes con EM, siendo 9,7% en PRE y 8,1% en POST [RR = 0,84 (IC95% = 0,44-1,58)]. El grado de aceptación de la recomendación farmacéutica se incrementó de 53,3% en PRE a 68,3% en POST [RR = 1,3 (IC95% = 1,02-1,62)].
Conclusiones: La integración del informe de monitorización farmacocinética en la historia clínica electrónica incrementa el grado de aceptación de las recomendaciones y puede disminuir la demora de las adecuaciones del tratamiento reduciendo los EM, mejorando con ello la calidad del proceso en eficacia y seguridad.
Palabras clave: Desarrollo tecnológico; Registros electrónicos de salud; Sistemas de información en salud; Farmacocinética; Errores de medicación; Innovación organizacional.
Abbreviations
MEs: Medication errors.
TECNO Group: Group for New Technology Assessment.
ECR: Electronic Clinical Record.
TDMR: Therapeutic Drug Monitoring Report.
OC: Orion Clinic®
RR: Relative Risk.
SEFH: Spanish Society of Hospital Pharmacy.
PhU: Pharmacy Unit.
ICTs: Information and Communication Technologies.
Contribution to scientific literature
This article is intended to state the importance of the inclusion of the Pharmacist activity in the comprehensive clinical record of patients, which will lead to an improvement in the quality of drug therapy, in terms of reduction of medication errors.
Introduction
Technological innovation is being implemented in the healthcare setting, leading to an improvement in the quality, safety and efficacy of patient care. The Group for New Technology Assessment (TECNO Group) of the Spanish Society of Hospital Pharmacy (SEFH), defines as new technologies applied to medication use: the software and hardware of the Hospital Pharmacy Unit (PhU) integrated with other hospital and patient databases, with the objective to share, standardize and unify the information within the Electronic Clinical Record (ECR). Both the Strategy Plan of the 2020 Group of the SEFH and the Regional Ministry of Health of the Community of Valencia have included the incorporation of new technologies within their lines of strategy1,2. These are used in medication selection, information and administration processes, including the record and assessment of the Pharmacist activities, integrated within the multidisciplinary teams for patient care3. Thus, the optimization of individualized drug therapy is encouraged, as well as the improvement in Hospital Pharmacy Care, and therefore, healthcare systems4,5.
There have been previous experiences in our setting with the use of computer systems for management of integrated processes, conducted in the area of clinical pharmacokinetics. The Hospital de Bellvitge has expressed that the computerized automatic integration of lab test and drug therapy data is necessary for planning the Therapeutic Drug Monitoring, including screening and an active and continuous re-assessment of treatments during hospital admission6.
According to the outcomes of the ADE Prevention Study, almost 2% of patients in the hospital setting will present adverse events caused by medications that can be prevented during their hospitalization; this fact increases the mean cost of hospitalization by 4,700 dollars7. The Medication Error (ME) rates vary among different studies. The rate obtained by the EMOPEM study ranged between 21.7 and 35.6%8. Lack of access to information about medications and lack of availability of information about the patient and lab test data at the time of prescription are acknowledged as possible causes of error9.
In this setting, clinical pharmacokinetics are essential for dose individualization and optimization of drug treatments, in order to reach maximum efficacy and safety10. The strategies to prevent and reduce MEs must be based essentially on interventions upon the systems, and new technologies can become tools for improving the use of medications, by increasing the access to information. Regarding Therapeutic Drug Monitoring in our centre, until January, 2012, the communication with the prescribing physician was conducted by sending the Therapeutic Drug Monitoring Report (TDMR) in paper format, in some cases with support by phone. When the ECR was implemented in the Health Department, it was stated that being able to view the TDMR and its availability in real time in the ECR would represent an added value, through an evolution note in the drug therapy follow-up of patients. Therefore, the objective of this study was to assess the impact of the inclusion of the TDMR in the ECR.
Material and methods
An observational ambispective cohort study conducted in a Public University Hospital (539 beds) with 1,800 annual applications for Therapeutic Drug Monitoring in hospitalized patients. In this setting, the implementation of ECR was initiated in 2007 with Orion Clinic® (OC), a system of comprehensive information for management of patient care and clinical processes, diagnostic tests, and treatments, in the Specialized Care Centres of the Conselleria de Sanitat (Regional Ministry of Health).
The study had a duration of 149 days, divided into two phases, randomly established and determined by the periods of implementation of the application: PRE (retrospective control cohort, between March and June, 2010: 49 days) with the TDMR issued in paper and sent through an internal pneumatic tube, and subsequently attached physically to the printed clinical record; and POST (prospective study cohort, from February to May, 2014: 100 days) with the TDMR issued in electronic format and included in the ECR as a clinical evolution note for the open episode, ordered chronologically according to patient care. The duration of each phase was determined with the aim to obtain similar sample sizes between both study arms.
The following were excluded: non-hospitalized patients, applications for Therapeutic Drug Monitoring by Critical Care and Newborn Units, and requests for monitoring with an objective other than dose adjustment.
The impact of including the TDMR in the ECR was assessed as the number of drug therapy adjustments made to treatments since the time of TDMR issue, number of MEs associated with said delay, and degree of acceptance of the TDMR. The variables collected were: number of TDMRs prepared, number of patients admitted to hospital with TDMR, number of adjustments made to treatments within a period over or under 24 hours from the time of issue of the TDMR, and MEs associated with said delay, as well as the degree of acceptance of the TDMR.
MEs were defined as any preventable incident which might harm the patient or lead to an inappropriate use of medications, when these are managed by healthcare professionals or patients / users11. The variables collected were: number of patients with MEs and number of MEs per each 100 TDMRs prepared. These were classified according to type of error: dose (higher, lower, extra, or missed), inadequate frequency of administration and/or duration of treatment (higher or lower) and their severity: potential errors (circumstances or incidents with the ability to cause an error, Category A) or without any harm caused (Categories B to D)11.
The repetition of the same ME in the same patient as a consequence of a delay in treatment update, according to the TDMR recommendation, was not considered an additional ME.
The source of information in the PRE phase was the drug therapy history in the Farmasyst® computer application with prescription by hand; while in the POST phase, treatment adjustments were confirmed by consulting the ECR in OC which includes the electronic prescription, pharmacist validation, and treatment administration. The list of patients with Drug Therapy Monitoring during the periods studied was collected through the pKWeb® application.
Statistical analysis was conducted with the STATA v12.0 package through relative risk comparison (RR; CI95%) of the PRE phase and the POST phase, and comparison of proportions through Square Chi Test Χ2). The study potency was calculated through the differences found in the variable "degree of acceptance of the Pharmacist recommendation".
Outcomes
During the period studied, 690 TDMRs were prepared in the Clinical Pharmacokinetics Unit, for 391 patients who met the inclusion criteria: 339 in PRE for 206 patients (mean TDMR per patient: 1.7 (CI95% = 1.4-1.9)) and 351 in the POST phase for 185 patients (mean TDMR per patient: 1.9 (CI95% = 1.7-2.1)). The drugs with the highest frequency of monitoring were: tacrolimus: 28.4% (n = 196), vancomycin; 23.9% (n = 165), digoxin 7.2% (n = 50), gentamicin: 7.2% (n = 50) and cyclosporine: 6.8% (n = 47), among others.
Table 1 shows the drugs monitored with the medication errors associated, according to the delay of modification and the phase of the study when they occurred.
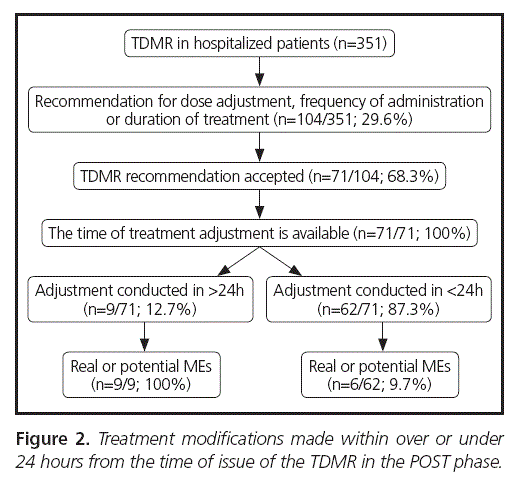
The adjustments made to treatment since the time of TDMR issue are detailed in Figures 1 (PRE phase) and 2 (POST phase). Differences were observed between the number of treatment adjustments made within less than 24 hours from the time of issue of the TDMR, with an increase from 73.9% (34/46) in PRE to 87.3% (62/71) in POST with a RR = 1.18 (CI95% = 0.97-1.43; Χ2= 3.38; p = 0.0659).
There were 35 MEs identified in 35 patients. The rate of ME was 9.7% (20/206) in PRE and 8.1% (15/185) in POST, with RR = 0.84 (CI95% = 0.44-1.58; Χ2= 0.31; p = 0.5804).
The number of MEs detected in PRE and POST was 5.9 and 4.3 per 100 TDMRs respectively, with RR = 0.69 (CI95% = 0.20-2.38; Χ2= 0.34; p = 0.5595).
The number of MEs in adjustments made within > 24 hours was 100% in both arms (12/12 and 9/9), while for < 24 hours it was 25.5% (8/34) in PRE and 9.7% (6/62) in POST, with RR = 0.41 (CI95% = 0.16-1.09; Χ2= 3.35; p = 0.0673). The distribution of the different types of errors was similar between the study arms.
Table 2 describes the classification of errors according to type of error and severity.
The degree of acceptance for Pharmacist recommendations increased from 53.3% (49/92) in PRE to 68.3% (71/104) in POST, with RR = 1.3 (CI95% = 1.021.62; Χ2= 4.63; p = 0.0314). The study potency estimated through the differences in said variable was 90.4%.
Discussion
In the year 2000, the report by the American Institute of Medicine recommended: to avoid trusting memory, to encourage the use of guidelines and linked processes, the simplification and standardization of processes, and the implementation of mechanisms which will allow learning and improving access to information in real time 12. For this reason, different national and international organizations are currently encouraging the use of information systems in healthcare institutions, with the aim to improve the quality, cost-efficacy and safety in patient care1.
As stated by our outcomes, the impact of including the TDMR in the ECR was an increase in the degree of acceptance of Pharmacist recommendations, reducing the time of delay for treatment update, and encouraging the involvement of Pharmacists in clinical decisions. This practice is in line with the mission of the TECNO Group, which encourages the incorporation of Pharmacist knowledge in new technologies, promoting effective, efficient and safe practices, with the highest quality, as part of comprehensive patient care2.
Published studies which have assessed the impact of Therapeutic Drug Monitoring recommendations in hospitals without ECR have reached the conclusion that 2.4 ± 1.4 days will go by between the first monitoring and the time when the written notification is sent, and 24% of these recommendations will be accepted13. However, acceptance studies based on on-line recommendations show that the degree of acceptance rises to 90.7%14. In this sense, the need for a higher access to information through ECRs is acknowledged; this has not been implemented yet in a great number of hospitals in our national territory15. The increase of the degree of acceptance of Pharmacist recommendations between both study cohorts probably involved an improvement in clinical practice, associated with the availability of information in real time.
A similar objective has been stated by the Primary Care district of North Jaén, where a communication platform between professionals in the district has been implemented; any doubt associated with the adequate use of medications can be posed and sorted out, with impact on 15,932 patients16.
The present study also stated that the availability of TDMRs in real time led to a reduction in MEs, though no statistically significant differences were observed, probably due to the low incidence of MEs identified; this could be a potential limitation of the study, which could have been overcome by enlarging the sample size.
Other authors who have conducted observational ambispective cohort studies in similar centres have assigned to the use of the integrated ECR the reduction of MEs of any type (not only associated with the Therapeutic Drug Monitoring process) from 66.5% to 55.2% (p < 0.007)17.
A systematic review conducted in 2013, which included 10 studies, highlighted the efficacy of the use of ICTs by multidisciplinary teams as one of the main strategies for detecting MEs and improving clinical practice18.
As the clinical manager of patients seen by the Conselleria de Sanitat centres, the objectives of OC are: to link all phases of pharmacotherapeutical process, to improve safety, to improve health outcomes, to promote the rational use and the efficient use of resources. One of the specific objectives of OC is to promote an active communication between the healthcare professionals responsible for patients, thus ensuring patient care traceability. OC is a technological advance that has identified opportunities for improvement in healthcare by analyzing each process, as well as the activity of all those involved and responsible for patient care. This has led to a deep cultural change in the management of healthcare activity; by optimizing and streamlining existing resources, it allows to build a new healthcare reality2. Processing and screening all information from the community population has generated the information necessary to direct an improvement in the NHS quality19.
According to the TECNO Group, new technologies must be evaluated before their implementation. Specifically in the clinical pharmacokinetic area, it must be assessed if the requirements established are met in order to manage dose individualization efficiently and safely, through Therapeutic Drug Monitoring20. However, this study was not designed for that aim, but to assess clinical outcomes in patients. Nevertheless, OC meets the essential requirements defined in the evaluation criteria established by the TECNO Group regarding user, software, patient, lab test data, and report; all these included in the ECR13. Thus, the ECR improves patient care training and quality, encouraging the transfer of knowledge, optimizing relationships between professionals, and encouraging decision making in an integrated manner, thus speeding up response times21.
Conclusions
The inclusion of the Therapeutic Drug Report in the Electronic Clinical Record increases the degree of acceptance of recommendations, and can reduce the time of delay for treatment adjustments, therefore reducing medications errors and improving the quality of the process in terms of efficacy and safety. An investment in new technologies will allow an improvement in patient safety, encouraging the communication between professionals.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Bibliography
1. Bermejo Vicedo T y Grupo TECNO. Papel del farmacéutico de hospital en las nuevas tecnologías en el sector sanitario. Farm Hosp. 2010;34:56-8. [ Links ]
2. Villanova P, Romero R, Pérez-Accino R, Alós M, Moya A. Orion Clinic. Un sistema de información orientado a transformar el uso de la información en la práctica clínica, administrativa y asistencia! de los hospitales. IS Informática y Salud 2011;85:18-26 (citado 30-04-2015). [ Links ]
3. Anoz L, Codina C, Sanjurjo M, Arranz A, Poveda JL, Martín I, et al. Grupo de Trabajo de Nuevas Tecnologías (Grupo TECNO) de la Sociedad Española de Farmacia Hospitalaria. Modelo de atención farmacéutica en el hospital, procedimientos normalizados de trabajo (monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2005 (citado 01/2016). Disponible en: http://sefh.es/sefhdescargas/archivos/protocoloaf.pdf. [ Links ]
4. Anoz, Codina, Sanjurjo, Arranz, Poveda, Martín, et al. Grupo de Trabajo de Nuevas Tecnologías (Grupo TECNO) de la Sociedad Española de Farmacia Hospitalaria. Plan Estratégico Grupo TECNO 2013-2017 (monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2005 (citado 01-01-2016). Disponible en: http://gruposdetrabajo.sefh.es/tecno/documentos/documentos/PE_TECNO_2013-2017_WEB_TECNO.pdf. [ Links ]
5. Sanjurjo M, Ribas J, coordinadores. Hacia el futuro, con seguridad. Grupo 2020 de la Sociedad Española de Farmacia Hospitalaria (monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2005 (citado 01-01-2016). Disponible en: http://gruposdetrabajo.sefh.es/2020/images/stories/documentos/archivos/objetivos_lineas_estrategicas.pdf. [ Links ]
6. Juvany Roig R, Leiva Badosa E, Cobo Sacristán S, Dastis Arias M, Tovar González E, Jodae Masanes R. Optimización de la actividad en el área de monitorización farmacocinética mediante un enfoque multidisciplinar integrado. 59o Congreso de la Sociedad Española de Farmacia Hospitalaria. Farm Hosp. 2014; (Supl. 1):149. [ Links ]
7. Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential drug events. Implications for prevention. JAMA. 1995;274:29-34. [ Links ]
8. Lacasa C, Ayestarán A y Coordinadoras del Estudio Multicéntrico para la Prevención de Errores de Medicación (EMOPEM). Estudio Multicéntrico español para la Prevención de Errores de Medicación. Resultados de cuatro años (2007 - 2011). Farm Hosp. 2012;36(5):356-367. [ Links ]
9. Leape LL, Bates DW, Cullen Dj; Cooper J, Demonaco HJ, Gallivan T elt al. Systems analysis of adverse drug events. JAMA. 1995;274:35-43. [ Links ]
10. Calvo MV, García MJ, Martínez J, Fernández MM. Farmacocinética clínica. Farmacia Hospitalaria, tomo I (monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2002 (citado 01-01-2016). Disponible en: http://www.sefh.es/bibliotecavirtual/fhtomo1/cap212.pdf. [ Links ]
11. NCC MERP Taxonomy of Medication Errors (Monografía en Internet). National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP); 1998 (citado 01-01-2016). Disponible en: http://www.nccmerp.org/sites/default/files/taxonomy2001-07-31.pdf. [ Links ]
12. Kohn LT, Corrigan JM, Donalson MS. To err is Human: Building a safer Health system. A report of the Committee on Quality of Health Care in America, Institute of Medicine. Washington DC: National Academy Press; 2000. [ Links ]
13. Triano García I, Matoses Chivirivella C, Maiques Llácer FJ, Rodríguez Lucena FJ, Del Moral Sánchez JM, Navarro Ruiz A. Impacto de las recomendaciones de monitorización farmacocinética. 57 Congreso de la Sociedad Española de Farmacia Hospitalaria (monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2012 (citado 01-01-2016). Disponible en: http://www.sbrafh.org.br/site/public/temp/5070783299b93.pdf. [ Links ]
14. Richard S Bourne, Chui Lynn Choo. Pharmacist proactive medication recommendations using electronic documentation in a UK general critical care unit. Int J Clin Pharm. 2012;34:351-357. [ Links ]
15. Lebrero García A, Gómez Pérez M, Ortega Moltalvan FA, Rueda Pérez C, Prats Olivan P, González Alfonso M. Impacto del informe farmacocinético en la seguridad del paciente. 57o Congreso de la Sociedad Española de Farmacia Hospitalaria (monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2012 (citado 01-01-2016). Disponible en: http://www.sbrafh.org.br/site/public/temp/5070783299b93.pdf. [ Links ]
16. Castillo Castillo R, García Hernández R, Toribio Onieva J R, Castro Campos JL, Rodríguez Toquero J, Dueñas Fuentes JR. Herramientas para la mejora de la calidad asistencial. XVII Congreso de Calidad Asistencial. Madrid: Sociedad Española de Calidad Asistencial; 2012 (citado 01-01-2016). Disponible en: http://www.hvn.es/servicios_asistenciales/ugc_medicina_preventiva/ficheros/comunicaciones_sadeca12.pdf. [ Links ]
17. Zlabek JA, Wickus JW, Mathiason MA. Early cost and safety benefits of an inpatient electronic health record. J Am Med Inform Assoc. 2011;18:169-172. [ Links ]
18. Lainer M, Mann E, Sönnichsen A. Information technology interventions to improve medication safety in primary care: a systematic review. Int J Qual Health Care. 2013;25:590-8. [ Links ]
19. Peiró Moreno S. Nuevos sistemas de información: posibilidades y limitaciones para la evaluación de la calidad asistencial. XXIX Congreso de Calidad Asistencial. Madrid: Sociedad Española de Calidad Asistencial; 2015 (citado 01-01-2016). Disponible en: http://calidadasistencial.es/wp-seca/wp-content/uploads/2015/02/2011_XIXX_Congreso_SECA_Murcia.pdf. [ Links ]
20. Anoz L, Codina C, Sanjurjo M, Arranz A, Poveda JL, Martín I, et al. Grupo de Trabajo de Nuevas Tecnologías (Grupo TECNO) de la Sociedad Española de Farmacia Hospitalaria. Monitorización farmacocinética. Plan Estratégico Grupo TECNO 2013-2017 (monografía en Internet). Madrid: Sociedad Española de Farmacia Hospitalaria; 2007 (citado 01-01-2016). Disponible en: http://sefh.es/sefhdescargas/archivos/fc.pdf. [ Links ]
21. De las Peñas MD; Samper P; Oruezabal MJ; Martínez-Amores B; García Menéndez C. Experiencia innovadora virtual en el funcionamiento de comités de tumores en el Hospital U. Rey Juan Carlos. XXXI Congreso de Calidad Asistencial. Madrid: Sociedad Española de Calidad Asistencial; 2015. [ Links ]
![]() Dirección para correspondencia:
Dirección para correspondencia:
Correo electrónico: saez_marbel@gva.es
(Marina Sáez Belló).
Recibido el 26 de octubre de 2015;
aceptado el 9 de mayo de 2016.











 texto en
texto en