My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Farmacia Hospitalaria
On-line version ISSN 2171-8695Print version ISSN 1130-6343
Farm Hosp. vol.40 n.6 Toledo Nov./Dec. 2016
https://dx.doi.org/10.7399/fh.2016.40.6.10607
Comparative study of preparation of hazardous drugs with different closed-system drug transfer devices by means of simulation with fluorescein
Estudio comparativo de preparación de fármacos peligrosos con varias modalidades de sistemas cerrados mediante simulación con fluoresceína
Eva González-Haba Peña1, Silvia Manrique Rodríguez1, Ana M.a Herranz Alonso1, Patricia Pérez Castán1, Mónica Moreno Gálvez1, Irene Iglesias Peinado2 and María Sanjurjo Sáez1
1 Pharmacy Service. Hospital General Universitario Gregorio Marañón, Madrid.
2 Faculty of Pharmacy. Universidad Complutense de Madrid.
ABSTRACT
Objectives: The level of environmental contamination generated during preparation and administration of hazardous drugs using different valve closed-systems and their combinations was compared. The actual impact on the overall time of preparation of cytostatics and the economic cost of the different modalities were also compared.
Methods: Comparative study of the preparation of fluorescein mixtures with different modalities of valve closed-system combinations. Environmental contamination was detected in critical points of connection, and in splashes produced at any other points. The main variable was qualitative detection of contamination by splashes through ultraviolet light when modalities with or without a connector were compared. A final number of 160 mixtures were prepared to detect differences of at least 5%.
Results: Splashes were produced in 7 preparations without a connector (p = 0.015). No significant differences (p = 0.445) were detected either in the use of a supporting vial spike vs an anchoring spike, or in the ChemoCLAVE® system vs valve systems with Fleboflex® solutions. Contamination at any critical point was produced in all preparations. The use of a supporting vial spike, syringe connector and bag solution with Luer connection was the most efficient modality.
Conclusions: A syringe connector is needed to guarantee a closed system. Anchoring spikes do not show higher advantages as compared with supporting vial spikes. Fleboflex® solutions with Luer bags are more efficient than ChemoCLAVE® and show similar safety. However, connections of these closed systems are not leak-tight, and it is therefore important to continue studies of contamination of the different closed system transfer devices.
Key words: Hazardous drug; Closed-system drug transfer device; Antineoplastic agents; Surface contamination; Drug preparation.
RESUMEN
Objetivo: Comparar la contaminación generada durante la elaboración y administración de fármacos peligrosos con diferentes componentes de sistemas cerrados y de manera secundaria, seleccionar el sistema más eficiente.
Material y métodos: Estudio comparativo de elaboración de mezclas de fluoresceína con diferentes combinaciones de sistemas cerrados de tipo valvular. Se consideró contaminación ambiental la detectada en los puntos críticos de conexión y las salpicaduras generadas en cualquier otro punto distinto. La variable principal fue la detección cualitativa mediante luz ultravioleta de contaminación por salpicaduras al comparar las modalidades con y sin conector. Se calculó un tamaño muestral de 160 preparaciones por modalidad, para detectar diferencias de al menos un 5%.
Resultados: Se produjeron salpicaduras en 7 preparaciones, todas sin conector (p = 0,015). No se encontraron diferencias entre utilizar punzón de apoyo o de anclaje (p = 0,445), ni entre el sistema ChemoCLAVE® vs sistema valvular con sueros Fleboflex®. En todas las preparaciones se produjo contaminación en algún punto crítico. La utilización de punzones de apoyo, conectores y sueros luer se ha identificado como la modalidad más eficiente.
Conclusiones: Es importante utilizar el conector de jeringa para que el sistema sea completamente cerrado. El uso de punzones de anclaje no parece presentar ventajas frente a los de apoyo y la combinación con los sueros Fleboflex® presenta una seguridad similar al sistema ChemoCLAVE®. Sin embargo, las conexiones de estos sistemas no son secas y, por tanto, es importante continuar con estudios de contaminación que comparen diferentes sistemas.
Palabras clave: Fármacos peligrosos; Sistema cerrado de transferencia; Antineoplásicos; Contaminación de superficie; Preparación de fármacos.
Contribution to scientific literature
The article offers a systematic comparison of different closed systems for handling of hazardous drugs. The main value of the research lies in the testing of compatible closed-system combinations which cover the whole chain of reconstitution, transfer and application of the pharmaceutical compounds.
The constant marketing of closed-system transfer devices for the safe handling of hazardous drugs makes necessary a continuous training of health professionals together with the evaluation of the features of the different systems. The evaluation of closed-systems in relation to contamination decrease has not yet been standardized and there are no recommendations about which closed-system to use.
Introduction
Occupational exposure to antineoplastic drugs is a concern for all professionals who are continuously involved in their preparation and administration due to their harmful mutagenic, carcinogenic, teratogenic and/or reproductive toxicity properties1. In the preparation of hazardous drugs (HD), biological safety cabinets (BSC) and personal protective equipment (PPE) are fundamental for handlers to ensure the lowest technically possible level of exposure2. Since its appearance, closed-system devices could also be essential to ensure this protection3,4.
Several well-known prestigious international organizations have stated the usefulness and recommendations of closed-system devices1,5-7.The National Institute for Occupational Safety and Health (NIOSH) has defined a closed-system drug transfer device (CSTD) as a system that mechanically avoids the transfer of environmental contaminants into the device and also the escape of high risk drugs or their aerosols.1 United States Pharmacopeia (USP) in its handling rules of hazardous drugs (USP 800) makes compulsory the use of closed-systems in both the preparation and administration of HD, when the dosage forms permit7. Several studies have shown the effectiveness of closed-systems to minimize environmental contamination8-14. Nevertheless, there are no specific tests to evaluate the criteria that should be met by these closed-systems.
The USP 800 recognizes the importance of studies of CSTDs and do not simply consider them as interchangeable systems. In the United States, the Food and Drug Administration (FDA) has established an ONB code for CSTDs that categorizes products to be used for safe handling. These CSTDs are destined for intravascular application and are defined as devices that, in healthcare, allow reconstitution and transfer of antineoplastic and hazardous drugs reducing exposition of healthcare staff (a particular device can be ONB only for some of the steps of the process, or for all of them).
In Spain, as in the rest of the European Community, there is no specific regulation about closed-system devices. In general, all of them are considered sanitary products regulated by the Real Decreto (Royal Decree) 1591/2009, and classified in class Ila. The available CSTDs are ChemoCLAVE® (ICU Medical Inc., San Clemente, CA), PhaSealTM (Becton, Dickinson and Company, Franklin Lakes, NJ), Texium® (Becton, Dickinson and Company, Franklin Lakes, NJ) connector, Smartsite®(Becton, Dickinson and Company, Franklin Lakes, NJ) valve, and Equashield®(Equashield LLC, Seaview Blvd. Port Washington, NY) PhaSealTM and Equashield® are the only system that have the ONB code that certifies that is really closed, but their use are not widespread in our country15,16.
In the last years, the use of ChemoCLAVE® has been extensively spread in Spain as the only available valvular alternative to the tree mode. ChemoCLAVE® is a valve system connecting one by one the different mixtures that comprise patients' treatment through "safe" connections and disconnections. The cytostatic is sent from the PS in a bag with a spike that it is not necessary to drain and that is connected, in the nursery unit, to an extension tube through a closed Luer male connection to the spike CLAVE® valve of the bag. This extension, in turn, connects with the pump delivery system available in the hospital by its irreversible adjustment to the spike of the infusion system17.
Most of the studies published about closed-system devices have been conducted with small samples sizes with poor statistical power18. In addition, there are few studies comparing different systems As far as we know, there are no published data with ChemoCLAVE®. There is only one study comparing ChemoCLAVE®, PhaSealTM and OnGuard® (B. Braun Medical Inc., Bethlehem, PA) using radioactive technetium. The authors found significantly more volume of leakage with ChemoCLAVE®. However, the study only analyzed the connection between vial and syringe, and the sampling methodology required touching the membranes connection. The vial access membrane of PhaSealTM is more inaccessible and this may affect the results19.
The main purpose of this work was to compare the level of environmental contamination generated during the preparation and administration of cytostatic drugs under actual working conditions using different valve closed-systems and their combinations. Other secondary objectives were to determine the actual impact on the overall time of preparation of cytostatics that have these systems and select the most efficient combination of components.
Methods
This comparative study on the preparation of fluorescein mixtures with different variants of closed-systems was conducted in a hospital that has a Pharmacy service (PS) with a central processing unit of cytostatic drugs that made, in 2015, a total of 50.695 antineoplastic preparations. The PS has an anteroom for the storage and preparation of the material, a passage room, and two clean rooms with three BSC class II type B for the preparation of chemotherapy. The clean room complies with ISO 14644-1: 1999.
The ChemoCLAVE® closed-system device was used in this study. This system is the combination of the bag spike with CLAVE with the Spiros® connector of the extension.. The vial spikes, syringe connectors and bag spikes used were from ICU Medical distributed in Spain by Hospira (Hospira Productos Farmacéuticos y Hospitalarios S.L., Alcobendas, Spain). Glucose solutions at 5% (GS5%) with a Fleboflex® Luer connection to be combined with the extension tube were also used. These materials were selected after a revision of all infusion solutions with Luer connections available in Spain, and because they were the only ones with a safety mechanic Robersite® (Halkey-Roberts Corp. Saint Petersburg, FL) valve20. Several combinations were analyzed aiming to compare:
- Safety during the preparation using a supporting vial spike vs anchoring spike. To do that, spikes with CLAVE connector of access to an air-filtering vial of 0.2 urn of ICU Medical were used, one universal and the other with an anchorage to vial of 20 mm.
- Safety during the preparation using a syringe without connector vs syringe with connector. The closed male connector used was Spiros® (ICU Medical Inc., San Clemente, CA).
- Safety during the preparation and administration of the ChemoCLAVE® valve systems vs a valve system combined with Fleboflex® solutions with Luer connection. The extension tube of ICU Medical that forms part of the ChemoCLAVE® system was used.
Fluorescein was selected as marker to measure contamination during the process of handling and administration. This marker allows visual detection as it becomes fluorescent upon exposure to ultraviolet (UV) light9.
Two different types of environmental contamination were considered21.
- Contamination of the critical points of connection (septum valve of the vial spike, syringe cone with or without connector, and valve of the infusion bag spike or valve of the infusion bag with Luer connection). This was considered a local contamination of low risk. Contamination of critical points was also checked after simulation of the administration.
- Contamination caused by splashing, detected at any point other than the critical points: vial, handler's gloves, work surface, etc. This was considered as a large and more variable contamination, and as a consequence, more difficult to control.
Sample size was calculated depending on the percentage of splashing or dripping in the groups with vs without connector. An absence of contamination (0%) in the group with connector was expected, whereas in the group without connector a low contamination of 5% was expected. Accepting an α risk of 5% and 80% power in bilateral contrast, 157 preparations were needed in each group to detect statistically significant differences between proportions of at least 5%.
Handlings were performed in BSCs, simulating actual work conditions. Two highly qualified nurses with similar experience and a pharmacist participated in the study. Both nurses had similar and wide experience in the handling of hazardous drugs. They handled the closed-system devices a year before starting this study. A total of 320 mixtures were combined by simulating a preparation of cytostatics using fluorescein vials. Each nurse prepared a total of 160 mixtures, 50% of each of the comparative branches. The final comparison includes 8 different combinations of closed systems that compare environmental contamination in the working area and in the critical points of connection. Forty preparations of each modality were made consecutively. A flow diagram of the comparative branches and the safety points analyzed is shown in Figure 1. The components used in the study are indicated in Figure 2.
In a previous step, 320 vials of 25 mg of fluorescein were prepared. Twenty five mg of powder fluorescein were weighted and introduced in amber glass vials of 50 ml. The glass vials were capped, sealed and labeled. To avoid external contamination with fluorescein, an external check with UV light was performed prior to the introduction in the BSC. Fluorescein mixtures were prepared in the BSC after cleaning the cabinet using alkaline soap and alcohol. A sterile cloth with an absorbent side up and a waterproof side down was placed in the cabinet. Each nurse made 160 fluorescein preparations and performed the following operations: placement of the spike in the fluorescein vial, reconstitution of the vials with 50 ml of physiological saline (concentration 0.05%)9, extraction of 40 ml of this solution in a 60 ml syringe, transfer to a 250 ml infusion bag of GS5% through the bag's safety valve or to the spike. Finally, administration was simulated by connecting the bag with the CLAVE or Luer valve to the extension that was previously connected to the pump system.
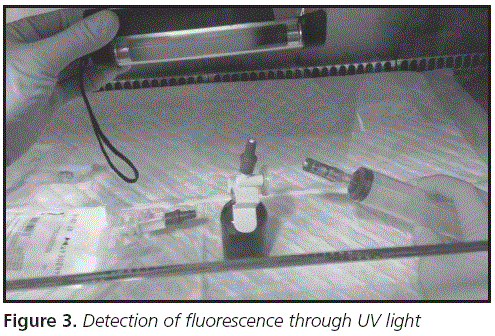
Fluorescein was detected by exposition to an UV lamp, UV light 365 nm (Cole-Parmer). After each preparation, the light of the BSC was switched off and the UV lamp was used to detect splashes in the working surface, latex gloves and handler's equipment (Figure 3). The pharmacist supervised the final mixture step of preparations and measured the fluorescence produced in each preparation.
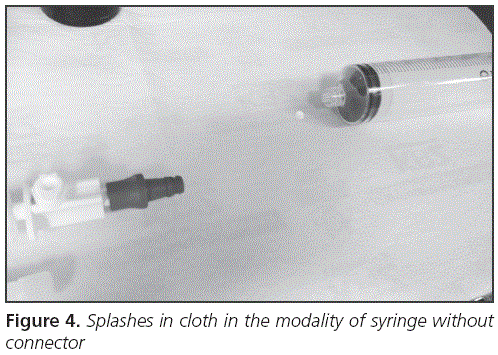
The main variable was the qualitative detection of environmental contamination due to splashes through fluorescein and UV light when the groups with connector vs without connector were compared. Groups with and without anchoring spike were also compared. Additionally, a quantitative measurement of the largest diameter of the droplet size originated during the manipulation was done with a metric ruler marked in millimeters. See for example Figure 4.
Contamination in critical points was also studied. From a qualitative point of view, it was considered contamination if any of the 3 critical points were visually present. A quantitative analysis was done by placing the points on cellulose paper and measuring the largest diameter of them. To detect safety after the disconnection, the valve of the extension tube was disconnected and a qualitative measurement of fluorescein on cellulose paper was then performed.
Other secondary variables were the time necessary to complete each modality of preparation and their economic cost. Time was measured by the pharmacist in each of the 320 preparations. Cost of each modality was calculated adding the different values (in #x20ac;) of the component of the closed-system. Two spikes were considered for each mixture. The additional cost of the Fleboflex® Luer bag instead of the Viaflo® (Baxter Healthcare Corporation, Round Lake, IL) solutions used with the CLAVE spike was taken into account. Prices used for solutions were the laboratory retail price plus taxes; for the rest of components, average prices in Spain according to Hospira were used.
Statistical analyses were performed with IBM SPSS Statistics for Windows, v21.0 (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). Results with p < 0.05 were considered statistically significant. Categorical variables were summarized through frequencies (presence or absence of contamination). Qualitative variables were assessed with central tendency measurements (size of the splashes in local contamination and time of preparation). Parametric variables were depicted by mean and standard deviation whereas the non-parametric ones were summarized with median and interquartile ranges 25-75 (IQR25-75).The statistical analysis of the primary endpoint was performed using Fisher's exact test. A Student t-test for independent variables was used to compare all parametric variables. Non-parametric variables were compared through the Mann-Whitney U test.
Results
The presence of qualitative environmental contamination, time and economic cost of the different closed-system models are recorded in Table 1. Local quantitative contamination in the critical points is detailed in Table 2.
Environmental contamination due to splashes and dripping was observed in only 7 of the 320 (2.2%) preparations. Two of these splashes (with a diameter of 0.2 and 4 cm) were in the modality 5 (without connector, with anchoring spike and bag spike with CLAVE connector), and 5 (with diameter values of 0.5, 1.0, 1.0, 1.5 and 3 cm) in the modality 7 (without connector, with supporting vial spike and bag spike with CLAVE connector). No other environmental contaminations were detected either in the cellulose cloth, in the gloves or the personal protective equipment of the handler. It is important to note that, in the seven preparations, splashes happened when the syringe was preloaded with fluorescein prior to transfer to the infusion bag, and all were without a syringe connector. The comparison of environmental contamination in preparations with vs without connector was statistically significant (p = 0.015). Comparison between the supporting vial spike and the anchoring spike was not significant (p = 0.445). Since all splashes happened before the introduction of the fluorescein into the bag, they are not related to the type of connection of the bag.
With regard to local contamination in critical points, in all preparations was observed, at some critical point, some kind of contamination but no differences among modalities were detected (Table 1). Concerning the size of the contamination in each critical point (spike, syringe, bag), the contamination observed in the spikes was similar among modalities whereas the contamination in the cone of the syringe and in the infusion point of the bag varied among modalities (Table 2).
The size of contamination in syringes with a connector was, on average, 0.1 cm higher than syringes without a connector. This difference was statistically significant (p = 0.000). With regard to contamination of the critical point of the bags, the point of access through a CLAVE valve showed a mean difference of 0.03 cm higher than the Luer bag (p = 0.017). In the administration step, no differences were found among modalities with ChemoCLAVE® vs modalities with Fleboflex® with Luer connection. In all cases, after disconnection, both the CLAVE and the Robersite® valves left a small drop on the cellulose paper (Table 1).
An increase in the time of preparation was observed when the anchoring vs the supporting vial spike was used (p = 0.036). Mixtures prepared with the CLAVE valve needed 16 seconds more preparation time than with a Luer bag (p = 0.000).
Modality 4 is the one that showed the highest effectiveness (supporting vial spike, connector and Luer bag). The cost of closed-systems for the preparation of HD is € 6.88 when the valve system in combination with Luer solution is used, and € 7.91 when the ChemoCLAVE® system is used (Table 1). Therefore, the introduction of solution bags allows a saving of € 1.03 per bag. An average of 3,161 treatments with infusion bags are performed monthly in our hospital, the economic impact of closed-systems is about € 300,000 per year. The introduction of Luer bags combined with the valve system of ICU Medical System could allow an annual saving of € 39,069.
Discussion
The evaluation of closed-systems in relation to contamination decrease has not yet been standardized and there are no recommendations about which closed-system to use7. Most studies attempting to show less environmental contamination with closed-systems are studies of surface contamination, with sampling techniques that allow evaluating residual contamination of cytostatic drugs. Other studies have used surrogate markers such as fluorescein, titanium tetrachloride and radioactive technetium18. NIOSH has recently proposed a protocol to determine the effectiveness of the closed containment systems to retain vapors22. Despite doubts about safety of filter-retention devices for drugs that may be vaporous4, their use in our country is common.
Our study has been performed with fluorescein, a marker that is not considered as particularly sensitive, but that is useful to detect drops and splashes during handling. In addition, it is a simple and inexpensive method and fluorescein is not harmful for the handler18. The UV source used in this study may have less sensitivity to detect spillages in comparison with other more intensive, and hence more expensive, UV sources on the market. This may be a limitation of the study.
One of our main findings is that the Spiros® connector of syringes is critical in considering the system as completely closed. Although the number of splashes was low even when this connector was not used, it is necessary to become aware of the importance of working in the optimum conditions to minimize the risk of exposure by reaching the lowest technically possible contamination level.
It has not been possible to show a higher theoretical safety of anchoring spikes because there has not been any accidental movement of the supporting vial spike resulting in spilling or splashes.
Concerning the use of Fleboflex® solutions during the preparation of the mixtures vs the bag spike with CLAVE valve, both methods have been equally safe. The risk of splashes did not increase. Additionally, its use has allowed a lower time of mixture preparation. In a previous survey about the use of solutions with Luer connexion, the handlers evaluated their use as highly positive. They are easy to handle because they avoid the repetitive movements of introducing the spike in the bag17. Another advantage is that the safety valve of solutions allows a higher injection flow20.
With regard to contamination of critical points, this study shows that the connections of these systems are not leak-tight. The presence of the connector increases contamination as compared with the syringe without connector (but diminishes the risk of splashes). CLAVE® connector slightly increases contamination as compared with Luer bag. However the measurement error of this contamination may be greater than the differences, and therefore these results should be interpreted with caution. This analysis has some limitations: it has not been validated, the detection limits are not known, it is not a sensitive method and the measurement error of contamination may be greater than the differences. However, it has been useful to highlight that these systems have a considerable level of contamination and may guide us about that level.
In the simulation of administration, there have not been differences between ChemoCLAVE® system and the combined system with Luer solutions. In both systems, there is a minimum residual adhered to the valve after disconnection. This local contamination is considered of lower risk than the probability of producing spilling or splashes during the preparation or administration of HD21. In the ChemoCLAVE® system, the Spiros® connector of the extension tube creates a vacuum when it is disconnected, sealing and closing the system automatically. It has been shown that occasionally it retains a residual volume of less than 0.07 uL in the end of the connector23 .In spite of the lack of specific studies supporting the use of Fleboflex® Luer solutions in the preparation and administration of cytostatic drugs, this study shows that safety for handlers of HD is similar to that of ChemoCLAVE® and its use allows considerable savings of money. The estimated cost associated with these devices provides a first approach to the economic impact that this technology might have on hospital's budget, although each institution will have to adapt it to their own activity and needs.
According to our results, connections of these closed systems are not leak-tight and none of these systems can be considered as totally closed, so it is necessary to continue working on improving the safety of handlers of HD with the use of these systems. To ensure the safety of health professionals working in the preparation and administration of HD, it is imperative that health systems employ significant resources in the use of CSTDs. It is therefore important to continue studies of contamination of the different CSTDs available on the market that allow us to select the most safe and efficient.
References
1. Howard J. Preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings. The National Institute for Occupational Safety and Health (NIOSH) Publication Number 2004-165, Cincinnati; 2004 (accessed 30 June 2016). Available at: http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf. [ Links ]
2. Fleury-Souverain S, Mattiuzzo M, Mehl F Nussbaumer S, Bouchoud L, Falaschi L, et al. Evaluation of chemical contamination of surfaces during the preparation of chemotherapies in 24 hospital pharmacies. Eur J Hosp Pharm 2015; 22 (6): 333-41 doi:10.1136/ ejhpharm-2014-000549 (published Online First: 22 April 2015). [ Links ]
3. Asociación Madrileña de Medicina del Trabajo en el Ámbito Sanitario (AMMTAS). Guía de buenas prácticas para trabajadores expuestos a agentes citostáticos. Madrid, Spain: Escuela Nacional de Medicina del Trabajo. Instituto de Salud Carlos III. Ministerio de Economía y Competitividad. 2014 (accessed 30 June 2016). Available at: http://gesdoc.isciii.es/gesdoccontroller?action=download&id=26/03/2014-199edf956b. [ Links ]
4. Nota técnica de Prevención 1.051: Exposición laboral a compuestos citostáticos: sistemas seguros para su preparación. Instituto Nacional de Seguridad, e Higiene en el Trabajo (INSHT); 2015 (accessed 30 June 2016). Available at: http://www.insht.es/InshtWeb/Contenidos/Documentacion/NTP/NTP/Ficheros/1043a1054/ntp-1051w.pdf. [ Links ]
5. ASHP Guidelines on Handling Hazardous Drugs. Am J Health Syst Pharm 2006; 63: 1172-91. [ Links ]
6. International Society of Oncology Pharmacy Practicioners Standards Committee. ISOPP standards of practice. Safe handling of cytotoxics. J Oncol Pharm Prac 2007; 13 Suppl: 1-81. [ Links ]
7. Hazardous Drugs: Handling in Healthcare Settings. General Chapter 800. 2014 (accessed 30 June 2016). Available at: http://www.usp.org/sites/default/files/usp_pdf/EN/m7808.pdf. [ Links ]
8. Wick C, Slawson MH, Jorgenson JA, Tyler LS. Using a closed-system protective device to reduce personnel exposure to antineoplastic agents. Am J Health Syst Pharm 2003; 60 (22): 2314-20. [ Links ]
9. Spivey S, Connor T. Determining sources of workplace contamination with antineoplastic drugs and comparing conventional IV drug preparation with a closed system. Hosp Pharm 2003; 38 (2): 135-9. [ Links ]
10. Harrison BR, Peters BG, Bing MR. Comparison of surface contamination with cyclophosphamide and fluorouracil using a closed-system drug transfer device versus standard preparation techniques. Am J Health Syst Pharm 2006; 63 (18): 1736-44. [ Links ]
11. Nygren O, Olofsson E, Johansson L. Spill and leakage using a drug preparation system based on double-filter technology. Ann Occup Hyg 2008; 52 (2) :95-8. [ Links ]
12. Siderov J, Kirsa S, McLauchlan R. Reducing workplace cytotoxic surface contamination using a closed-system drug transfer device. J Oncol Pharm Pract 2010; 16 (1): 19-25. [ Links ]
13. Sessink PJ, Connor TH, Jorgenson JA, Tyler TG. Reduction in surface contamination with antineoplastic drugs in 22 hospital pharmacies in the US following implementation of a closed-system drug transfer device. J Oncol Pharm Pract 2011; 17 (1): 39-48. [ Links ]
14. Clark BA, Sessink PJM. Use of a closed system drug-transfer device eliminates surface contamination with antineoplastic agents. J Oncol Pharm Pract 2013; 19 (2): 99-104. [ Links ]
15. Gómez-Álvarez S, Porta-Oltra B, Hernández-Griso M, Pérez-Labaña F, Climente-Martí M. Evaluación de dos sistemas cerrados en el proceso de elaboración de quimioterapia parenteral. Farm Hosp. 2016; 40 (1): 36-43. [ Links ]
16. Forte Pérez-Minayo M, Castillo Bazán E, Hernández Segurado M, Arias Moya MA, Pelegrín Torres P, Bécares Martínez FJ. Use of closed systems in the Hospital Pharmacy. Farm Hosp. 2016; 40 (2): 102-117. [ Links ]
17. González-Haba E, Manrique-Rodríguez S, Herranz A, Casado C, Sánchez MN, Sanjurjo M. Evaluation and selection of closed-systems for safe cytostatics handling. Eur J Clin Pharm 2015; 17 (4):279-288. [ Links ]
18. Power L. Closed-System Transfer Devices. For Safe Handling of Injectable Hazardous Drugs. Pharmacy Practice News. 2013 (accessed 30 June 2016). Available at: http://www.pharmacypracticenews.com/download /cstd_ppn0613_wm.pdf. [ Links ]
19. De Ausen L, DeFreitas EF, Littleton L, Lustik M. Leakage from closed-system transfer devices as detected by a radioactive tracer. Am J Health Syst Pharm 2013; 70 (7): 619-23. [ Links ]
20. Agencia Española de Medicamentos y Productos Sanitarios. Fichas técnicas. Fleboflex salina fisiológica grifols solución para perfusión. Madrid; 2013 (accessed 30 June 2016). Available at: http://www.aemps.gob.es/cima/pdfs/es/p/65528/P_65528.pdf. [ Links ]
21. Favier B, Labrosse H, Gilles-Afchain L, Cropet C, Perol D, Chaumard N etal. The PhaSeal® system: impact of its use on workplace contamination and duration of chemotherapy preparation. J Oncol Pharm Pract 2012; 18 (1): 37-45. [ Links ]
22. CDC. A Vapor Containment Performance Protocol for Closed System Transfer Devices Used During Pharmacy Compounding and Administration of Hazardous Drugs. 2015 (accessed 30 June 2016). Available at: http://www.federalregister.gov/articles/2015/09/08/2015-22525/a-va por-containment-performance-protocol-for-closed-system-transfer-devices-used-during-pharmacy. [ Links ]
23. Kelly J. The Role of Closed System Transfer Devices in Mitigating the Risks Posed to Healthcare Workers in the Handling of Hazardous Drugs. Entropy Research; 2011 (accessed 30 June 2016). Available at: http://www.biokon.gr/datafiles/file/The%20Role%20of%20Closed%20System%20Transfer%20Devices%20in%20Mitigating%20the%20Risks.pdf. [ Links ]
![]() Correspondence:
Correspondence:
Correo electrónico: eva.gonzalezhaba@salud.madrid.org
(Eva González-Haba Peña).
Recibido el 18 de julio de 2016;
aceptado el 14 de septiembre de 2016.