Contribution to scientific literature
This article evaluates the efficiency of using cisplatin/pemetrexed, cisplatin/gemcitabine/bevacizumab or carboplatin/paclitaxel/bevacizumab as first line treatment for patients with non-squamous non-small-cell lung cancer, from the perspective of the hospital management. The interest of this article lies in the fact that, as far as we know, no pharmacoeconomic evaluation has been conducted so far in order to evaluate the efficiency of using the main three first line treatment regimens for this condition, taking also into account the potential economic impact of the imminent launch of generic pemetrexed. Given our current economic situation, with limited healthcare resources, this type of studies appear as an additional tool to be considered at the time of making decisions, by complementing the information about efficacy and safety extracted from clinical trials.
Introduction
After colorectal cancer and prostate cancer, lung cancer is the most frequent tumour in Spain, with around 27,000 new cases /year (13% of the total number)1; 80-85% of these are non-small-cell lung cancer (NSCLC) cases2. The majority of patients are diagnosed in advanced stages of the disease3, and for this reason it is one of the oncologic conditions with higher mortality rates (21.4% mean survival at 5 years)4.
The standard treatments for NSCLC are platinum-based antineoplastic regimens5, and in recent years, new agents have been incorporated for metastatic disease, such as pemetrexed and bevacizumab. Some regimens based only on chemotherapy doublets have also been described for the induction stage, followed by maintenance with pemetrexed; but guidelines currently recommend the incorporation of pemetrexed or bevacizumab since the induction stage6. Both drugs have demonstrated an improvement in the life expectancy of these patients, when used in treatment regimens including one platinum-based induction stage with pemetrexed or bevacizumab, followed by maintenance with any of these two drugs. These regimen are mostly: cisplatin/pemetrexed (Pem/Cis)7, cisplatin/gemcitabine/bevacizumab (Gem/Cis/Bev)8 and carboplatin/paclitaxel/bevacizumab (Pac/Carb/Bev)9. The improvement in efficacy represented by incorporating these two new drugs has also entailed an increase in the cost of treatments9. And the fact that the efficacy outcomes shown by the three regimens are similar, together with the lack of clinical trials comparing them with one another, means that currently it is difficult to pick the most efficient regimen. Therefore, particularly in our current economic situation, with limited healthcare resources, it is necessary to conduct a cost-effectiveness analysis to be used as a tool for decision making at the time of optimizing those resources available.
Therefore, the objective of this study was to conduct an economic analysis to estimate the cost-effectiveness ratio of first line antineoplastic regimens for NSCLC treatment, from the perspective of the hospital management.
Material and methods
Pharmacoeconomic model
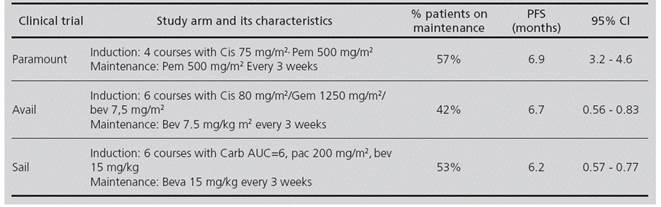
A mathematical model for cost-effectiveness was prepared, based on a decision tree with three branches, each of them representing the cis/pem, cis/gem/bev and carb/pac/bev regimens (Figure 1). The probability values for each transition were obtained from these Phase III clinical trials: PARAMOUNT7 (cis/pem); AVAIL8 (cis/gem/ bev) and SAIL9 (carb/pac/bev).
These treatment regimens are based on an induction stage followed by a maintenance stage on monotherapy which is continued until disease progression (Progression Free Survival - PFS), or unacceptable toxicity.
4 Cis (75 mg/m2)/Pem (500 mg/m2) courses, followed by pemetrexed (500 mg/m2) as maintenance every 3 weeks.
6 Gem (1250 mg/m2)/Cis (80 mg/m2)/Bev (7.5 mg/ kg) courses, followed by bevacizumab (7.5 mg/kg) as maintenance every 3 weeks.
6 Pac (200 mg/m2)/Carb (AUC = 6)/Bev (15 mg/kg) courses, followed by bevacizumab (15 mg/kg) as maintenance every 3 weeks.
A summary of the main characteristics of the PARAMOUNT, SAIL and AVAIL clinical trials appears in Table 1.
The economic model was validated by an expert panel of oncologists, with the objective to clarify any differences and/or contradictions described in scientific literature. The Microsoft Excel 2013® computer application was used for developing the model. Figure 1 shows a schematic diagram of the decision analysis.
Estimation of efficacy and costs
The efficacy variable used was Progression Free Survival (PFS) as described in the clinical trials for each treatment. Given that in the AVAIL study, those patients who survived the induction stage were randomized to receive bevacizumab as maintenance at a 7.5 mg/kg or 15 mg/ kg dose, and no statistically significant differences were found between both bevacizumab doses, the 7.5 mg/kg dosing was used for cost estimation.
The pharmacological cost was estimated according to the LSP (Laboratory Selling Price) of the medications studied, resulting from applying the profit margins established on the retail price-VAT of the Catalog by the General Council of Official Pharmaceutical Colleges, and with the relevant 7.5% deduction on the LSP established by Royal Decree 8/2010 and provided by the Ministry of Health, Social Services and Equality on October, 201210,11 (Table 2).
Table 2 Costs per unit used in the pharmacoeconomic model for 1st line NSCLC treatment (at October, 2015 euros)

G.M.P. Generic Medicinal Product
* 7.5% reduction on the LSP established by Royal Decree 8/201014
For dose calculation, we used the anthropometric characteristics of a standard patient representative of Spanish oncology patients12: weight of 70.15 kg, body surface of 1.78 m2, and serum creatinine of 0.6 mg/dL. The administration of carboplatin in order to obtain the desired area under the curve (AUC) was determined by the Glomerular filtration rate (GFR). Calvert Formula was used in this study to calculate the carboplatin milligrams needed for each administration13. The total pharmacological cost for each oncological regimen was estimated by assuming maximum use of vials. All costs included in the analysis were stated in 2015 euros (€).
Time horizon, analysis perspective, and discount rate
The time horizon considered was the time until progression to first line of treatment, which coincided approximately with 1 year of modelling based on clinical trial outcomes. The study was conducted from the perspective of the hospital management, taking into account only the direct costs for drug acquisition. Given the limited survival of patients (none of the oncology regimens went beyond one year of treatment), it was not adequate to apply a discount rate correction(14.15).
Base case and Sensitivity Analysis
The mean values of costs and efficacy were considered for the base case in the study.
In order to confirm the robustness of the base scenario outcomes and the consistency of the estimations, various univariate deterministic sensitivity analyses were conducted, considering the following factors:
Improvement in efficacy (PFS). The PFS of patients who survive the induction stage is not specified in clinical trials; therefore, it was estimated with the following formula:
The model assumes that the PFS of patients with progression during the induction stage is equal to the duration of said stage. This assumption is based on the fact that, in clinical practice, imaging tests to confirm the status of the disease are usually conducted after completing the induction stage. Therefore, the estimated PFS for patients who presented disease progression during the induction stage was:
2.8 months for the cis/pem regimen (4 induction courses every 3 weeks).
4.2 months for the pac/carb/bev and cis/gem/bev regimens (6 induction courses every 3 weeks).
A sensitivity analysis was conducted, assuming a 20% improvement in the proportion of patients who survived the induction stage for each regimen (Table 3); by applying the previous formula in an overall PFS for each regimen, this would result in:
cis/pem: 7.69 months
cis/gem/bev: 7.18 months
pac/carb/bev: 6.58 months
Table 3 Incremental cost-effectiveness ratio and sensitivity analysis for the cost-effectiveness analysis (at October, 2015 €) of 1st line NSCLC treatment

a Incremental cost-effectiveness ratio per additional month of Progression Free Survival
b An alternative is defined as dominated when there are options with higher efficacy and lower cost.
c An alternative is defined as dominant when it presents the highest efficacy at the lowest cost.
3. Modification of the acquisition costs for pemetrexed, assuming a 30% cost reduction with the launch of generic drugs.
Results
Base Case Result
The proportion of patients who moved on to the maintenance stage during clinical trials was: 57% for the cis/ pem regimen, 42% for patients who received gem/cis/bev, and 53% for the carb/pac/bev regimen. Based on our model, the mean cost of treatment per patient for the gem/cis/bev, cis/pem, and carb/pac/bev treatment regimens would be 15,594.74€, 19,442.01€ and 36,095.17€ respectively. The car/pac/bev regimen is the dominated alternative, because it is the most costly treatment regimen with the worst health outcomes. The difference in costs between the cis/pem and cis/gem/bev approaches is 3,847.21€, with an incremental cost-effectiveness ratio (ICER) per additional PFS month of 19,303€ (Table 3).
Sensitivity analysis in alternative scenarios
There was a robust outcome in the sensitivity analysis for all scenarios, considering the current drug acquisition costs; with an ICER of 9,720€ per additional PFS month for the cis/pem regimen, when a 20% increase in efficacy is considered for all treatments. However, with a 30% reduction in the price of pemetrexed, the cis/pem alternative would become the dominant alternative, because it presents the best health outcomes at a lower cost. The best incremental cost-efficacy ratio for the cis/pem treatment occurred when a 30% discount was assumed for pemetrexed, as well as a 20% increase in efficacy (ICER = -3,974€/additional PFS month ).
The carb/pac/bev regimen was the dominated alternative in all scenarios studied (Table 3).
Discussion
The outcomes of our model show that, from the perspective of hospital management, the carb/pac/bev regimen is the dominated alternative in first line treatment for NSCLC, because there are current regimens with higher efficacy and lower cost; therefore, in our opinion, this option would be relegated only to patients who cannot tolerate cisplatin-based treatments; for example, those patients with underlying renal conditions. At the current acquisition cost for the drugs studied, the cis/ gemc/bev treatment is cheaper than the cis/pem treatment, with an incremental cost of between 8,217 and 19,303€ per additional PFS month. Typically, a therapy will be considered cost-effective if its ICER is below the predefined threshold of acceptability17. If no explicit thresholds of acceptability are available, a therapy can be considered cost-effective if its ICER is within the range of other therapies which healthcare managers have been willing to pay for16. In a study published in 2013 about the efficiency of oncologic treatments in Spain, Oyagüez et al. found that the ICER values per additional PFS month were within the range of 5,979.87 to 46,716€17 for first line therapies; therefore, according to these studies, the conclusion could be that the cis/pem treatment is a cost-effective strategy.
Besides what has been previously stated, the incorporation to the market of generic pemetrexed (Alimta®Eli Lilly Nederland B.V, date of authorization: September, 2004) can lead to a significant change in our current situation. With a 30% reduction in the current acquisition costs for pemetrexed, the cis/pem treatment would become the dominant alternative (the best health outcomes at a lower cost) for the 1st line treatment of NSCLC patients. These outcomes should be taken into account at the time of making decisions in order to optimize the resources available.
The interpretation of the outcomes of this analysis must be understood as an approach to the efficiency of oncologic regimens, and in any case it should be considered alongside a series of limitations. The main limitation is that only the pharmacological cost of treatment regimens has been considered, though some therapies could be associated with additional costs for the health system, such as those derived of their administration, monitoring, or management of adverse events experienced by patients and caused by medication. The approach chosen for this analysis, considering the pharmacological cost only, intends to equalize the outcomes for the different regimens evaluated. Therefore, the outcomes of this pharmacoeconomic analysis should be confirmed through effectiveness records, comparing the use of healthcare resources for the therapeutic options evaluated, in the setting of daily clinical practice. Besides, we must not forget that bevacizumab presents a series of important limitations that are usually frequent in this type of patients, because it is contraindicated in haemoptysis cases, or when the tumour is adjacent to a major thoracic vessel. Meanwhile, according to the outcomes of this model, the conclusion that can be reached is that the cis/pem treatment is cost-effective, and that the launch of generic pemetrexed could turn it into the dominant 1st line treatment option for NSCLC patients.











 texto en
texto en