Introduction
Chronic Kidney Disease (CKD) is a public health problem at global level1-3. It is estimated that around 20% of the >60-year-old population suffers advanced CKD, with a significant number of undiagnosed patients, either due to lack of renal function monitoring, or because they suffer a hidden CKD4.
There will be an increasing number of persons with CKD who will need to undergo treatments with side effects at short, medium and long term5. The main reasons for this are:
The inability by patients to excrete drugs that are eliminated through the kidneys can lead to a build-up or metabolites, in case of repeated administration.
In nephropathy cases with hypoalbuminemia, drug effects of higher intensity can develop as a consequence of a reduced binding to plasma proteins and the subsequent increase in the free circulating fraction of the drug.
Reduction of the therapeutic effects of some drugs such as thiazide diuretics.
According to the reports by the Colombian Association of Nephrology, one of ten adults in the world suffers some renal damage condition, and the rate of patients under replacement therapy (dialysis and transplant) increased in 15% per year during the past decade6.
In order to ensure patient safety as a component of quality of care7, Pharmaceutical Care Practice (PCP) must be conducted as a clinical activity within Pharmaceutical Care (PC), with the objective of reducing the morbimortality associated with the use of medications, through activities performed by the Pharmacist, who will be responsible for identifying the needs of the patient regarding pharmacological treatment, with the aim to achieve results that will improve the quality of life of patients, working together with the healthcare team in order to make decisions about the treatment initiated, to ensure its safety and efficacy8.
Within PCP, we must highlight the identification, prevention and solution of Negative Outcomes associated with Medication (NOMs), which are considered events that can affect patient health due to the use of medications9-11; and all this is caused by the presence of one or more Drug-Related Problems (DRPs), which are circumstances or events that lead or can lead to the development of a NOM.12-14
The prevalence and incidence of CKD which requires renal replacement therapy has increased progressively in Colombia; currently there are approximately 20,000 persons under renal replacement therapy, which represents a prevalence of 450 patients per one million inhabitants. The incidence is approximately of 5%; and this population could be doubled during the next 10 years, and reach a prevalence superior to 800 patients per one million inhabitants.15,16
In Barranquilla, the prevalence of CKD is of approximately 46 persons per 100,000 inhabitants1. During 2013, the year when the study was conducted, 5,904 patients were admitted in hospital departments other than the Intensive Care Unit (ICU); 295 (5%) of these patients were seen in a Renal Unit, with 91.53% hospitalized patients and 9.26% outpatients. Due to the reduction in the glomerular filtration rate, the excretory and waste depurative function is reduced; this situation can affect the drug kinetics and dynamics, and this can have an impact on pharmacological treatment, an increase in the intensity and therapeutic effect, such as adverse events17. Therefore, the Pharmacist, as a medication professional, can offer interventions that will benefit the health and wellbeing of patients with CKD. According to studies, Clinical Pharmacy has ensured an increase in the knowledge of medications by patients, a reduction in hospitalization rate, and an improvement in their quality of life18,19.
The present study was conducted in order to understand the types of drug-related problems, and the negative outcomes associated with medications, that can be detected, prevented and solved in patients with chronic kidney disease, through pharmaceutical care practice.
Materials and method
The design was Quasi-experimental, so that variables would not be intentionally modified, but interventions could be conducted that modified the final out-come of pharmacological treatment in patients. It was Descriptive, because the characteristics of the variables associated with the PF process were determined in patients with CKD, through the detection, prevention and solution of DRPs and NOMs and pharmaceutical interventions; and regarding time, it was Retrospective.
NOMs can be classified into three types: Necessity NOMs, when patients are not receiving an adequate treatment for their condition, and the other cause is that the health problems of patients are not receiving treatment; Effectiveness NOMs, when the treatment does not meet the therapeutical objectives, based on the dose administered; and Safety NOMs, identified when health problems develop as a consequence of drug therapy, which can be dose-dependent or not.
The variables were classified as dependent: DRP, NOM, types of Pharmaceutical Interventions and their clinical outcomes; and independent: demographics (age, gender and cause of CKD); the clinical variable was CKD stage.
In order to detect DRPs, there was an assessment of the general data of the patient, the medications administered, adherence to prescribed treatments, altered lab test results, habits, allergies to certain drugs and/or foods, physical activity, condition such as renal impairment, and other factors that can modify the patient-medication relationship. The selection and justification for use of medications was assessed; this way, Necessity NOMs were determined. Effectiveness NOMs were reviewed on the basis of the doses used and patient evolution, and Safety NOMs were detected through a comprehensive search in the Clinical Record about the presence of any ADE (Adverse Drug Event) by analyzing the different treatments, identifying any potential drug interactions, a subsequent pharmaceutical interview, and the evolution of their health situation.
The variables for gender (male, female), mean age (years) and cause for CKD were obtained through the review of clinical records and interviews with patients and relatives.
Statistical Analysis
The outcomes of the pharmaceutical interventions in the study were statistically analyzed with the STATA program version 12.0. A descriptive analysis was conducted with central tendency and dispersion measures for quantitative variables, and relative frequencies for qualitative variables. Student’s t-test and Square-chi test were applied to analyze NOMs and p<0.05 was determined for statistical significance
Patients were handed an Informed Consent for accepting or not. The sample was formed by 18 to 90 year old patients with CKD diagnosis, on polymedication, defined as patients under pharmacological treatment with over 5 medications20, hospitalized and also outpatients, managed during nine months. The Ethics Committee of the centre approved the study.
The study was conducted in a High-complexity Clinic with Renal Unit Departments, a high-complexity Pharmacy Service, Renal Transplant Unit, and Coronary and Neurological Intensive Care Units.
The study was based on the DADER Method for PF, which includes 9 stages: service offering, first interview, assessment form, study stage, assessment stage, intervention stage, results of the intervention, new assessment and subsequent interviews.
Results
In total, 47 patients accepted to enter the PCP program; 21 of them were men and 26 were women. Regarding age groups, 8 patients were in the 18-to-44-year-old range, 18 patients were in the 45-to-64-year-old range, and 21 patients were classified in the 65-to-88-year-old group. In terms of ethnicity, 13 patients were white, 5 patients were black, and 29 were of mixed race.
Based on their level of disease evolution, there was 1 patient with CKD Stage I; 4 patients with CKD Stage II; 17 patients with CKD Stage III; 5 patients with CKD Stage IV; and 20 patients with CKD Stage V in hemodialysis phase.
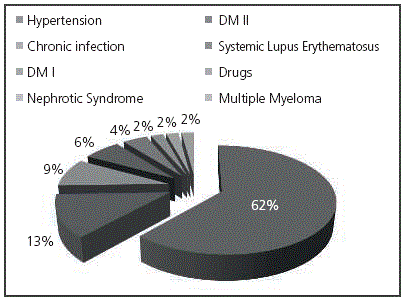
The primary etiology in all CKD patients appears in Figure 1.
All patients in CKD Stage III were >60-year-old and it should be expected that the main causes were chronic diseases such as hypertension, Diabetes Mellitus and chronic infections.
For patients with CKD Stage V, the main etiology was hypertension (45%), similarly to Stage III, followed by Diabetes Mellitus Type 2 (20%), chronic infection (15%), Systemic Lupus Erythematosus (10%), Nephrotic Syndrome and Diabetes Mellitus Type 1 (5 % each).
When comparing the causes for CKD in Stages III and V, it was observed that Diabetes Mellitus Type I (DMI) and chronic infections appeared in both.
An average 7.32 ± 3.74 medications were prescribed to patients with CKD; these drugs were grouped according to their ATC codes.
The highest prescription was for Group C, Cardiovascular System, with 172 medications; this means that patients with CKD are administered a higher number of medications acting upon this physiological system. This was followed by Group A, Alimentary Tract, with 115 prescriptions, and Group B, Blood and blood forming organs, with 113 prescriptions; these two groups were the most widely used in all patients, due to their prophylactic nature.
In total, there were 7 Necessity NOMs, 23 Effectiveness NOMs and 21 Safety NOMs.
The analysis of NOMs showed that those patients with CKD included in the PF program were at risk of presenting 1.09 ± 1 NOM, with a Mode of 1 case per the total number of patients. The patient with the highest number of NOMs presented 5, while the lowest number of NOMs was 0.
The NOM subtype with the highest prevalence was Non-Quantitative Insecurity, with 21 cases, followed by Quantitative Ineffectiveness, with 14 NOMs. Those with a lower proportion were: Non-Quantitative Ineffectiveness, with 9 cases, Non-Treated Health Problem, with 5 cases, and Problem by Unnecessary Medication, with 2 cases. There were no cases of Quantitative Insecurity.
Similarly to NOMs, 51 DRPs presented in total; this indicated that for each DRP presented (cause) there appears a NOM (consequence). See Table 1.
Table 2 shows the types of PF conducted in patients with CKD.
Figure 2 and Figure 3 show the analysis of the Pharmaceutical Interventions conducted and not accepted for each type of NOM, as well as the health problems solved.

Figure 2 Analysis of the Pharmaceutical Interventions conducted in each type of Negative Outcomes Associated with Medication and Health Problems solved.

Figure 3 Analysis of Non-accepted Pharmaceutical Interventions in each type of Negative Outcomes associated with Medication and Health Problems solved.
In total, 12 dose re-adjustments were conducted, and 10 of these were accepted. The medication with the highest number of dose adjustments was cefepime, with 3 cases, followed by ampicillin + sulbactam, bisacodyl and metoclopramide with 2 cases each. The medications with one single case were clobazam, ceftriaxone and dexamethasone.
Discussion
There was no significant difference found in CKD patients regarding gender; that is to say, male patients are at the same risk of developing it as female patients. However, age is a factor that increases the risk of developing CKD, in agreement with the study by Salvador González B. et al21, which analyzed the prevalence of Chronic Renal Disease (CRD) in 40 Primary Care Centres in the urban area of Barcelona, where CRD prevalence is higher when age increases.
On the other hand, hypertension and DM are the most important risk factors that lead to deterioration in renal function, because the kidney is one of the target organs in these conditions; at the same time, together they will increase the cardiovascular risk22,23.
The highest number of prescriptions presented in Group C, Cardiovascular System; and therefore, closer monitoring must be conducted for CKD. Treatment is conservative, controlling risk factors as well as treating and preventing their reversible causes. This was followed by Group A, Alimentary Tract, and B, Blood and blood forming organs, because there must be prevention and treatment for metabolic alterations such as malnutrition and anaemia, among others24-26.
Necessity NOMs showed that not all the health problems are being treated in these patients; that is to say, only their basal disease was controlled, while the problems caused by it were being overlooked.
Effectiveness NOMS were those with the highest proportion. Though patients were being treated with the medication for their basal disease, this was not yet controlled, and this triggered a higher likelihood of presenting other NOMs, and a higher risk to their health. Besides, in our study, there were more reports for Quantitative Ineffectiveness; the reason for this was the lack of modification in treatment dosing, even when this was allowed by the therapeutic window of drugs.
Patients with CKD present a higher risk of developing undesirable events such as ADRs (Adverse Drug Reactions), because this condition can alter drug kinetics27. Safety was the NOM with the highest number of reports in terms of Non-Quantitative Insecurity, which means that in this case, dosing was not a factor triggering this NOM.
DRPs showed the reason for all this NOMs presented: the likelihood of adverse effects and dosing were the main axle, because these led to a higher increase in NOMs, increasing the risk of mortality in these patients. Therefore, these points must be emphasized at the time of treating their basal conditions. Pharmacovigilance could help to prevent these two types of DRPs, not only through identification but also by helping in their solution; on the other hand, interactions and the wrong administration of the medication were problems that presented but that can be prevented with continuous education, unlike those personal characteristics that are individual factors, but can be mentioned to patients so that they will take them into account in the future, in order to prevent new NOMS.
The likelihood of adverse effects was the main reason for NOM presentation. This differs from the study by Alviz et al., where Pharmacotherapy Follow-up was conducted during hospitalization to 49 patients who had undergone transplantation at the Fundación Clínica Valle del Lili (Cali, Colombia); 14 DRPs were detected, and 77.8% of these were for Ineffectiveness of Pharmacological Treatment28.
All these problems were detected and solved, pharmaceutical interventions were conducted verbally and in writing, with the aim of helping to improve the quality of life of patients, and therefore prevent their development.
Chemello C. conducted a study about pharmaceutical care practice in patients with Chronic Kidney Disease, where 29 DRPs were detected before the intervention by the Pharmacist in 21 patients; from these, 18 patients presented one DRP, while 3 patients presented two or more DRPs. After the intervention, the number of DRPs was reduced to 9 in 8 patients in total, which means that 22 patients did not present DRPs again, and 68.9% of the initial DRPs were solved29.
This shows that the work by the Pharmacist is as important as that of any other health professional; they will not only be in charge of the adequate storage and preparation of medications, but will also contribute to meeting their target objectives with the lowest risk possible, as described by Ohnishi et al. in their “Study about the Importance of the Pharmacist regarding the Haemoglobin Levels in Patients with Renal Anaemia”. Pharmacists offered recommendations to physicians, particularly regarding changes in erythropoietin doses and the administration of iron preparations. This advice by pharmacists led to a significant reduction in the haemoglobin levels of the higher group (12 g/dL), and significantly higher in the lower group (10 g/dL). On the other hand, there was an increase in the haemoglobin levels in the optimal group. These findings suggest that the active involvement of Pharmacists in the treatment of renal anaemia for patients under hemodialysis had a great therapeutic impact.30
Dose readjustments were conducted because treatments were not effective and safe, through pharmaceutical intervention.
One of the options to prevent NOMs is through education, which was a key factor for developing this study, because it generated higher trust among health professionals, patients, and the Pharmacist.
Conclusion
The NOMs with the highest prevalence were those for Non-Quantitative Insecurity; therefore, these represent a highly prevalent problem. This shows the importance of this public health problem, and its major clinical, social and economic consequences, which are associated with Chronic Kidney Disease. Therefore, it is necessary to involve all healthcare professionals and patients, in order to obtain as a result a better use of medications, to prevent NOMs, and to reduce the morbidity associated with drug therapy.











 texto em
texto em