Introduction
Multiple Sclerosis (MS) is a demyelinating disease of the Central Nervous System (CNS), which will typically present initially as flares with different symptoms that vary based on the location and extent of the episode. Over the years, in approximately half of the patients, the disease evolves from flares to a progressive stage which causes an increasing level of disability, with a major impact in the quality of life and productive capacity of patients1.
Symptoms are very diverse, because the nerves in any part of the brain or the bone marrow can be affected; therefore, there can be visual disorders, walking alterations, sensitive disorders, and urinary problems, among others1. This condition is one of the most frequent causes for disability in young adults. It presents numerous consequences, and mobility reduction is one of the most negative aspects both at psychological and physical level.
During the past 20 years, researchers have made great advances in MS treatment, due to their increased knowledge about the immune system and the ability of using Magnetic Resonance Imaging (MRI) to monitor the disease. Until some years ago, the therapies approved by the European Union Agency (EMA) for MS treatment were disease modifying medications (immunomodulators and immunosuppressants). In recent years, the pharmaceutical industry has conducted research for the development of new molecules that can improve the symptoms caused by MS, and thus improve the quality of life of patients2. The new drugs for the improvement of MS symptoms (tetrahydrocannabinol / cannabidiol and fampridine) are some of the latest achievements for patients with this disease. Fampridine represents a major advance: it is the first drug marketed for improving one of the most disabling symptoms presented by MS patients: walking disability, which over 70% of patients report as a symptom of their disease. The Spanish Agency of Medicines and Medical Devices (AEMPS) authorized its marketing in 2012.
Fampridine (4-aminopyridine) acts by blocking potassium channels, reducing the leakage of the ion flow through these channels, and thereby prolonging repolarization and enhancing action potential formation in demyelinated axons and neurological function. Presumably, by enhancing action potential formation, more impulses might be conducted in the central nervous system, thus improving walking speed, and at the same time reducing the sensation of rigidity and spasticity3.
The objective of this study was to evaluate the efficacy and safety in adult patients who initiated treatment with fampridine for walking improvement, through the 25-foot walk test (T25FW) and the 12-item multiple sclerosis walking scale (MSWS-12).
Methods
A descriptive retrospective study was conducted, including all patients diagnosed with MS who presented walk impairment and had initiated treatment with fampridine between March, 2014 and February, 2015. The dose was administered according to the product specifications: one 10mg tablet every 12 hours (one tablet in the morning and one tablet at night).
The inclusion criteria established in the protocol were: Relapsing-Remitting MS (RR-MS), Secondary Progressive MS (SP-MS) or Primary Progressive MS (PP-MS), presenting walk disability; >18-year-old patients, with creatinine clearance > 80 ml/min, without previous history of epileptic episodes, and no treatment with inhibitors of type 2 organic anion transporters (OATs-2).
Walk disability was evaluated through the Expanded Disability Status Scale (EDSS), and those patients with a final score from 4 to 7 were included.
The following variables for each patient were collected, after review of the electronic clinical record from SERGAS (IANUS®): gender, age, type of MS, concomitant immunomodulator therapy (interferon beta, glatiramer acetate, or natalizumab), disease duration, EDSS score, T25FW test and MSWS-12 scale. These two latter items were evaluated before treatment initiation, at 14 days and at 3 or 6 months in patients who had completed this period at the time of data analysis.
The T25FW test quantifies lower limb function and mobility, and consists in walking a 25-foot (7,625 m) course and timing it. The patient is placed in one end of the course, and must walk as quickly as possibly, but safely. The time is calculated from the initiation of the instruction to start and ends when the patient has reached the 25-foot mark4. The MSWS-12 questionnaire consists of 12 questions about the walking limitations caused by MS during the last two weeks, and patients must answer on a 1 to 5 score, where 1 is not at all, 2 is a little, 3 is moderately, 4 is quite a lot, and 5 is extremely.
The efficacy criteria considered were: ≥ 20% increase in speed in the T25FW test, and ≥ 6 point reduction in the MSWS-12 scale. These clinical benefits had to be observed in the first visit after treatment initiation (week 2); in this case, patients were considered responders, and fampridine treatment was continued. There was a new evaluation at month 3 or 6, and if this clinical improvement was not present, patients were considered non-responders, and treatment had to be discontinued.
Regarding safety, it was reviewed if patients had suffered any of the most frequent adverse effects described in the pivotal phase III clinical trial: urinary tract infection, falls, insomnia, headache, asthenia, dizziness, nausea, backache, balance disorders, upper respiratory tract infection, arthralgia, nasopharyngitis, and paresthesias5.
The SPSS® program was used for the statistical study, conducting a descriptive analysis for all variables. Qualitative variables were analyzed by calculating absolute and relative frequencies (number and percentage), and quantitative variables were studied through mean and standard deviation.
Outcomes
The number of patients who met the inclusion criteria for the study was of 6. Table 1 shows their demographical data, as well as diagnosis and treatment data and variables associated with the disease, and the breakdown of outcomes into responder and non-responder patients.
Table 1 Demographic, diagnostic and treatment data, and variables associated with the disease
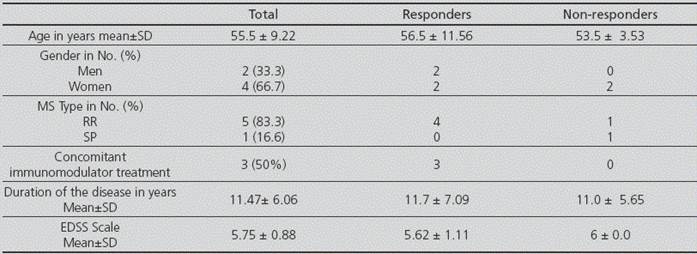
MS: Multiple Sclerosis; SD: Standard Deviation.
RR-MS was the most prevalent type; on the other hand, none of the patients had a PP-MS diagnosis. Three of the patients were receiving concomitant immunomodulator treatment; this number only represents 6.7% of those MS patients on immunomodulator treatment who are monitored in our Pharmacy Care Unit.
The first evaluation of drug efficacy was conducted for all patients at 14 days of treatment initiation. The response rate to treatment with fampridine according to protocol has been 66.7% (4 out of 6 patients). The second test was conducted in the four responder patients at 3 or 6 months since treatment initiation according to visit. The improvement obtained in the first review was sustained in the MSWS-12 scale, and even improved in the T25FW test (Table 2).
Table 2 Results in the T25FW test and MSWS-12 scale at baseline, at 14 days and at 3-6 months
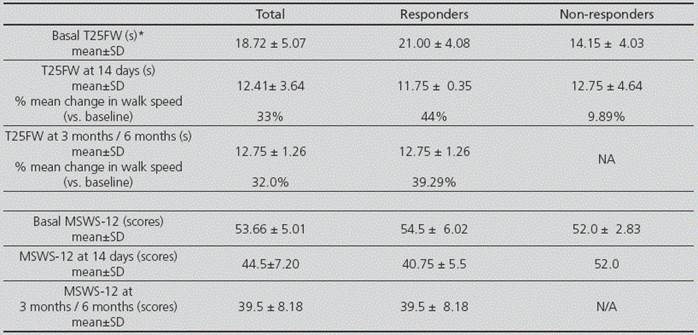
T25FW: 25-foot walk test; MSWS-12: 12-item mobility scale; *s=seconds; SD: standard deviation; N/A: not applicable
Regarding safety, only one patient presented adverse effects of mild intensity and without any impact on him, who did not need to discontinue treatment for this reason; three of these adverse effects had been described in the pivotal clinical trial (nausea, headache and nasopharyngitis), and another one had not been collected (diarrhoea).
Discussion
MS affects more women than men; with an approximate 2:1 incidence ratio, though some data suggest that this ratio is even higher6. This proportion has been found in our study population, and both the mean 55-year-age of patients and the proportion by gender (33.3% men vs. 66.7% women) is similar to the data obtained in the clinical trials used for the authorization of fampridine by the EMA5,7.
Disease progression is a typical characteristic in PP-MS and SP-MS6. In our case, the percentage of patients differs from those in the pivotal clinical trials MS-F2035 and MS-F2047, with a higher number of patients with RR-MS (83.3% vs. 27% and 35.8%) and a lower number of patients with SP-MS (16.6% vs. 55% and 51,7%). However, the time of evolution of the disease since diagnosis is quite similar (11.5 years vs. 13.4 and 14.4 years). In our series, only 50% of patients were on treatment with some disease modifying drug, and this is lower than the 66% and 69.2% from the pivotal clinical trials and higher than the 30.8% in a German study8.
Currently, the most widely used way to evaluate walk in clinical practice is the T25FW test, included since 1994 within the Multiple Sclerosis Functional Composite to evaluate functionality in MS9. One of the questions we must ask is whether this test, used to assess the efficacy of fampridine both in clinical trials and in our case, has sufficient clinical relevance for its outcomes to be associated with an improvement in the ability to walk, quality of walk, resistance, and duration of effect. According to different studies, a ≥20% improvement in walking speed is considered clinically relevant, and correlates with improvements in the perception of the ability to walk (MSWS-12)10.
In the clinical trials conducted by Goodman et al., fampridine has demonstrated an improvement of around 25% in walking speed according to the T25FW test, in those patients with response to the drug 5,7. In our case, outcomes were slightly superior, with a 44% improvement at 14 days, and 39.6% at 3-6 months. In the timed test, those patients without response at 14 days presented a better basal outcome (14.15 s ± 4.03 vs. 21.00 s ± 4.08).
In pivotal clinical trials5,7, the “ad hoc” analysis of aggregated data showed that those patients with response at the T25FW test (≥20%) also presented response at the MSWS-12 (≥6). In our study, responder patients for the T25FW test criterion were also responder for the MSWS-12 questionnaire, because they achieved an improvement of 13.75 ± 0.52 points at the first control, and 15.5 ± 2.16 at their second and third visit; these outcomes were quite higher than the 6 points required as the cut-off point to consider that there was response to treatment. The score in the MSWS-12 questionnaire at the initial visit of our patients was slightly inferior than that from patients included in pivotal clinical trials (53.66 ± 5.01 vs. 70.7 ± 18.6 and 73.8 ± 17.8). These data are interesting, because in our study there were worse basal outcomes in the T25FW test than in the studies conducted by Goodman5 (1.3 feet/s vs. 2.1 feet/s); however, the results from the MSWS-12 questionnaire were better. The perception of improvement in daily life that had been observed in pivotal studies was also achieved in our patients, with an approximate reduction by 6 and 14 points, respectively, in the group of responder patients, and 0.45 and 0 in non-responders.
Prugger and Berger conducted a study in 67 patients with MS11, previous to the requirement by the EMA to re-evaluate at 2 weeks. They obtained a 32.8% efficacy in the T25FW test at 4 weeks of treatment, and 16.4% at 6 months; in both cases, their data are lower than ours. On the contrary, a French study conducted with 112 patients with MS12 , and a Slovenian study conducted with 30 patients13, obtained a 50.9% and 56.7% response rate, respectively, in the T25FW test at 14 days of treatment initiation; these data are superior to those obtained in our study. In other Spanish studies not published but presented as communications in national congresses, the efficacy of fampridine measured by the T25FW test at 14 days of treatment is quite dissimilar, with response rates from 68% to 100%14; in all cases with response rates very superior to those obtained in pivotal clinical trials (34.2% and 42.9%); however, all the sample sizes in these studies were <30 patients.
Regarding drug safety, only one patient presented adverse effects, and none of them was serious: three had been described in the pivotal clinical trial (nausea, headache and nasopharyngitis), and another one not described (diarrhoea). Finally, this patient discontinued treatment of his own free will, and after completing the study, even though he met all criteria for continuing treatment at all control visits, because he reported that he did not notice any real improvement. In the study by Prugger and Berger11, three patients experienced adverse effects that led them to treatment discontinuation, two for nausea and one for insomnia. In the French study12, 65 patients presented at least one adverse effect, though the researchers did not consider that they were severe, and seven patients discontinued their treatment.
The authorization for fampridine marketing in Spain is on conditional approval; this means that more information is expected about this medication, specifically about its benefits beyond its effects on walk speed, and about an early identification of responder patients, therefore it is important to continue analyzing the data obtained in real clinical practice 3.
The main limitation in our study is its reduced sample size. The outcomes achieved are hopeful, both in efficacy and in safety, particularly because fampridine is the only drug currently authorized by the AEMPS and the EMA for controlling such a disabling symptom as instability while walking.











 text in
text in 


