Contribution to scientific literature
It is necessary to have an adequate availability of antidotes in those healthcare settings with Emergency Care, because sometimes their early administration might determine the prognosis of the poisoned patient. Hospital Pharmacy Units are responsible for ensuring the availability of antidotes in the hospital setting; however, this is a complex matter, because some factors can determine their availability, such as the frequency of a specific type of poisoning, the urgency of antidote administration, difficult acquisition, the high cost of some of them, and their short expiration period.
The attached manuscript presents the development and functioning of a computer tool that will be helpful for the pharmacists and physicians of different hospitals, in order to improve the availability of those antidotes with higher availability issues. This is an application that allows the fast location of those hospitals where a specific antidote is stocked, and to speed up its loan if necessary.
Though different international and national studies have suggested the creation of this type of application as a possible solution for the issue of antidotes availability, very few experiences have been published about this, and none of them as an initiative by the same hospitals managing the antidotes. The most similar publication in literature is the initiative by the Italian National Centre for Toxicological Information, which has information and control on the antidotes stocked in different places in the country.
Introduction
Acute poisoning and abuse drug overdoses represent 2-3% of the Emergencies managed at hospitals. Most of them are mild exposures that can be treated with symptomatic measures only. However, in some patients it might be necessary to administer an antidote in order to minimize morbimortality risks, and in these cases, treatment success will depend on the availability and sufficient quantity of the antidote required at the place of patient care.
Numerous national and international publications have stated that the required antidotes are quite often not available in those hospitals managing poisoning. The first studies on antidote availability date from the 90s. The Dart et al. group had already reported in 19961 the insufficient stock of eight antidotes in the Pharmacy Units from 137 hospitals in Colorado, Montana and Nevada. Also in Spain, an article published in 1998 by Nogué et al.2 stated the lack of homogeneity in terms of antidote availability, and in 2006, Aguilar et al.3 showed that Catalan hospitals did not stock all those antidotes required in order to treat any poisoning, and that these deficiencies were both qualitative and quantitative, and affected hospitals of all levels of care.
The availability of antidotes in the different settings of care is a complex matter. Some factors can determine their availability at the points of care, such as the frequency of presentation of a specific type of poisoning in a geographical area, the urgency for antidote administration, the difficulties for its acquisition, the high cost of some of them, and their short expiration period. Hospital Pharmacy Departments are responsible for the acquisition and custody of antidotes, as well as for ensuring their availability when required. However, there are no rules regarding which antidotes must be included in hospital formularies, and in which quantity; therefore, their stock will depend on the involvement by physicians and pharmacists in each centre4.
In this context, the Antidote Work Team from the Societat Catalana de Farmàcia Clínica (SCFC) was created in 2013, formed by four pharmacists from hospitals in Catalonia with different levels of complexity, and two clinical toxicologists with wide experience in acute poisoning, both in adult and paediatric patients. The main objective of this team was to establish updated recommendations about the antidotes that should be stocked in hospitals, and in which quantity, according to the level of care complexity; as well as to review and update the toxicological indications for each one, and the recommendations with the highest level of consensus about dosing for adults and children5. The team was aware of the difficulties in order to implement these recommendations in hospitals, taking into account the cost represented by keeping the adequate stock of some antidotes. For this reason, it was decided to design a virtual network of antidotes between hospitals, that allowed to locate on-line those hospitals that stocked those antidotes with the highest difficulty in terms of availability, and at the same time ensured that the medication was loaned in case of necessity. Therefore, the Pharmacy Departments could hold a minimum stock in order to cover the first hours of treatment for poisoned patients, and it would be possible to complete treatment by requesting a loan of the antidote from a nearby hospital. This network has been functioning since July, 2015, and this article describes its functioning and initial outcomes.
Material and methods
A computer application was developed with the assistance of a company with experience in the development of computer tools and web-pages associated with the healthcare setting. For this development, there was funding by the SCFC. The four starting points for developing the network of antidotes were the selection of the antidotes to be included, the hospitals involved, the control of the stock of antidotes, and loan management.
Antidotes
Initially, the work team conducted a bibliographic review of the scientific evidence on the use of antidotes for treatment of poisoning. A final list was prepared, including 34 medications recommended to be available in any high-technology hospital, as well as in regional hospitals of reference; it would be desirable that 22 of these medications were available in any hospital, regardless of their level of care. There was also a calculation of the quantity that would be advisable to have of each one, based on the complexity of each hospital.
Based on this initial list, there was a selection of those drugs that would become part of the antidote network, giving priority to factors such as low use, high cost, and/or difficulties in availability. Said difficulties could arise because these were foreign medications, magistral formulations, or because the required doses to cover the treatment for the toxicological indication were much higher than the rest of usual indications, and therefore, the quantity available in hospitals was not enough. Those antidotes that presented more frequent deficiencies in the studies published on availability were also identified. The final result was the selection of 15 antidotes to be included in the network: anti-digoxin antibodies, methylene blue, deferoxamine, dimercaprol, edetate calcium disodium, ethanol, physostigmine, fomepizole, glucagon, hydroxocobalamin, pyridoxine, pralidoxime, silibinin, antibotulinum serum, and antiophidic serum.
Hospitals involved
The creation of the Antidotes Network was informed to all Catalan Hospital Pharmacy Departments, in order to create awareness about the project, and to request the involvement by all public and private Catalan hospitals. An application form for joining the network was attached, requiring the incorporation of a pharmacist of contact (“farmatox”) and a physician from the Emergency Unit of referral (“urgetox”). Data from the Pharmacy Department and Emergency Unit were also requested, as well as the address of the centre and the GPS latitude and longitude coordinates, if known.
Control of the antidote stock and management of loans
An application was designed that allowed to create virtual formularies of those antidotes included in the network, so that each hospital involved could record the entry and exit movements of the antidotes, and therefore ensure that the stock was the real one at any time. In case of loan request, the application also created the relevant movements, and generated an email to the requesting hospital as a notification.
In order to facilitate a fast and easy location of the antidotes available in the hospitals included in the network, a system of search by medication or hospital was designed, using the on-line map applications Google Maps and Google Earth.
Results
The Antidote Network in Catalonia started functioning on July, 2015. It can be accessed through this on-line platform: www.xarxaantidots.org. This application features an area open to all internet users, with general information about the project, the centres included, documents prepared by the team, links of interest, and the option to conduct non-urgent toxicological consultations to the team experts. Thirteen queries had been received until June, 2016, and these were relative to the specific treatment of some poisoning, the review of the contents of antidote formularies, and information on the availability and problems with antidote supply.
The private area of the application can be accessed by all hospitals that have voluntarily joined the antidote network, with a username and a password. Four profiles were created for each participant hospital: “farmatox”, “urgetox”, a general Pharmacy user, and one for the ER, which allow to access the application when the first ones are not working. All profiles allow to locate antidotes and request loans, but only the “farmatox” can record the entry and exit movements of medications in the application, in order to keep the stock updated. A licence was also created to access the Toxicological Information System from the National institute of Toxicology and Forensic Science and the Spanish Foundation of Clinical Toxicology, by express request of these organizations, and in order to facilitate their job in terms of resolution of queries.
By June, 2016, there are 40 public and private hospitals from the four provinces in Catalonia that have joined the Antidote Network (Table 1).
Table 1 Catalan hospitals included in the Antidote Network

N/A= Data Not Available.
* Data provided by the Departament de Salut of Catalonia.
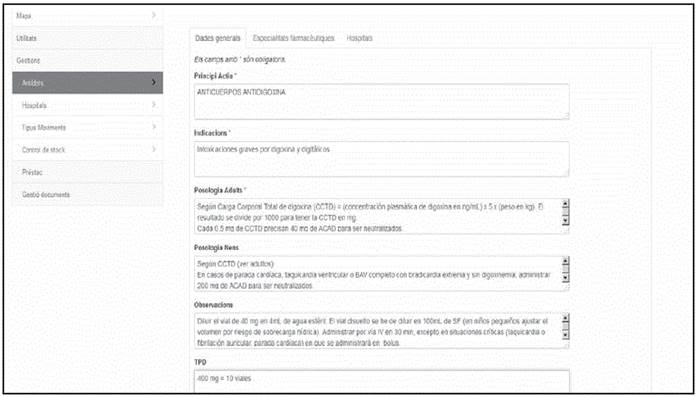
The private area is divided into four sections. The Antidote Section contains information about the antidotes, that can be consulted on-line. This is a dynamic list, maintained by the members of the group, that collects data about the toxicological indications, dosing with the highest consensus both for adults and children, formulations available, observations about the administration, stability, adverse reactions, and other considerations to be taken into account, as well as the quantities recommended to be stocked according to the complexity of each hospital (Figure 1).
The section for Antidote Stock Management collects the quantity available of the antidotes in the network stocked in each hospital. The application allows the “farmatox” to enter any entry and exit movements. Each entry must include: medication, number of units, lot, expiration date, and type of movement. For the latter, two types of entry movements have been defined (purchase of medication and return of the loan to other hospital), and three types of exit movements (own use, expiration, and loan) (Figure 2).
The pharmacist responsible in each hospital must conduct manually all movements for stock maintenance (loan movements as well as for own use). In order to facilitate this stock maintenance, the application allows printing a document with the units entered from each lot, and the expiration date for each one. Those units expired will appear highlighted in red.
The application allows to conduct the search of antidotes by medication or by hospital, in the section of the map showing the information of all hospitals included in the network. When the search is conducted by hospital, the following data can be consulted in the map: names of the “farmatox” and “urgetox”, address, telephone number, e-mail, fax, and working hours of the Pharmacy Department and telephone number of the Emergency Unit. All antidotes available in the centre are also displayed, with their number of units and the next expiration date (Figure 3). When conducting the search by antidote, all hospitals where it is available will be displayed, as well as the number of units available, and the next expiration date (Figure 4).
Once the centre for requesting the antidote has been located, the application allows the generation of a loan. In order to do this, the requesting hospital must select the medication requested, its quantity and to which hospital, from the Loan Section. Once this is completed, the application will automatically send an e-mail to the “farmatox”, the “urgetox” , and the general e-mail address of the Pharmacy Unit receiving the request. However, it is recommended to make a phone call in order to ensure the request has been received. A document is also generated in the fax format typically used to request loans between Hospital Pharmacy Units (Figure 5). When the application is accessed, the hospital receiving the request will see that there is a pending application for loan. They can reject it, accept to loan the total number of units requested, or modify the number of units they can loan. Once accepted, the application will generate the relevant use.
Finally, the Documentation Section contains all materials that the team prepares about use and availability of antidotes. The documents incorporated include two guidelines (one for the “farmatox” and another for the “urgetox”), explaining the network functioning in detail and through graphs.
During the first year since the network was implemented, the private area has been accessed 2,102 times, and nine loans between hospitals have been conducted. In two cases, these were due to poisoning by Amanita phalloides affecting various members of the same family, where the hospitals only had silibinin available for the first hours of treatment, and therefore requested a loan to another hospital. In both cases, the poisoning occurred in the province of Girona; in the first case, the regional hospital requested six vials of silibinin to the hospital of referral in the province, and in the second case, this latter hospital requested eight vials to a high-technology hospital in Barcelona. The third case was about several patients intoxicated by the smoke of a home fire; the regional hospital from the province of Barcelona that managed the patients requested the loan of a vial of hydroxocobalamin to a nearby regional hospital included in the network. The fourth case was a Grade II poisoning by snake bite in an 11-year-old boy. The patient was administered one vial of antiophidic serum at the hospital, and another vial was requested as a loan to a nearby hospital, in case a new dose was required; fortunately, this was not necessary. Two more loans of antiophidic serum were requested by a high-technology hospital in Barcelona to hospitals of referral in the province. The last case was a botulinum poisoning that affected eight persons: all the antibotulinum serum units available in Catalonia had to be set in motion (three loans).
Discussion
It is necessary to guarantee an adequate stock of antidotes in those hospitals managing poisoned patients; however, this is not always easy. The administration of antidigoxin antibodies, for example, can save the life of a patient with severe digoxin poisoning, but numerous studies have stated that not all hospitals stock this antidote, or a sufficient quantity of it. There are various reasons for this lack of availability: it is an expensive antidote, indicated for a non-frequent severe situation, with a relatively short expiration period, and up to 20 vials could be necessary to treat an adult patient. In such cases, the antidote network is an efficient tool to ensure availability and to reduce the stock necessary in hospitals. On a recent review of the recommendations for availability5, the antidote group established a stock of only 10 vials of anti-digoxin antibodies in high-technology hospitals and of reference at province level, based on the recommendation for initial administration of 50% of the estimated dose of antibodies, followed by response assessment after two hours, in order to decide whether the administration of the rest of the dose is necessary or not6. By including this antidote in the network, the Pharmacy Unit initiating treatment would have some leeway to obtain the rest of the treatment from a nearby hospital, thus guaranteeing treatment and reducing by half the expenses for maintaining a stock of this medication at hospital.
To create a database with the updated antidote stocks accessible to all hospitals managing hospital emergencies is not a new idea. The New Zealand hospitals already suggested its creation as a solution for the deficiencies found7,8. Along the same line, the Centro Antiveleni di Pavia - Centro Nazionale di Informazione Tossicologica9 created the Banca Dati Nazionale degli Antidoti (BaNdA), based on a study of antidote availability at the Italian Emergency Units. This is an on-line platform that allows to find updated data about the qualitative and quantitative availability of antidotes in all hospital units involved, to search for a specific antidote by city or region, and to access all contact details required in order to request a loan. Within the first five years after its implementation, 20 different antidotes were requested10. Also through the initiative of French Antitoxic Centres, the Banque de Sérums Antivenimeux (BSA) was created, which collects eight antiophidic serums for the treatment of poisoning by approximately 30 of the 160 species of venomous snakes registered11.
The case of the Antidote Network of Catalonia is the first experience based on the initiative from the same pharmacists and physicians at the hospitals managing poisoned patients. The number of antidotes included is much lower that in the BaNdA, and it has been restricted to those antidotes with higher difficulties in terms of availability, with the objective of simplifying their management and encouraging the participation of hospitals. One of the limitations of the Antidote Network is that it is not possible to connect it to the hospital systems for electronic prescription or stock management; therefore, the success of this project will depend on the direct involvement by the “farmatox” in terms of updating their stock movements.
The list of antidotes included in the network will change according to the needs of its hospitals, any epidemiological changes in poisoning, the launch of new antidotes, and problems for supply of others. In fact, antidotes initially included, such as methylene blue and pyridoxine, are no longer part of the network as of June 2016, because an adequate availability in hospitals has been observed, and these will be replaced by others such as glucarpidase or idarucizumab.
Other antidotes that might be included in the Antidote Network are antiophidic serums for bites by exotic venomous snake bites. The presence of this type of snakes in people’s homes, zoos or travelling exhibitions has become a reality in Spain and other European Union countries, as well as the severe accidents generated, and the need for antivenom agents of difficult availability. The Antidote Network can help to solve this problem.
The problems of drug shortages, frequent during the past months, can have consequences on the efficacy, safety or cost of treatments, and affect drugs from different classes. Some antidotes have been affected by failures in the production and supply chain12,13. The impact of drug shortage can vary according to the availability of other alternative options and the duration of the supply problem. One of the drugs included in the Antidote Network is fomepizole, which has presented supply problems since 2010. In cases like this, as well as for drugs with difficult acquisition (for example, an tidotes that must be imported as foreign medications), the network can play an important role and facilitate access. In fact, in a positioning document on the impact of the problems of antidote shortage published by the American College of Medical Toxicology and the American Academy of Clinical Toxicology12, it is recommended to implement regional strategies to facilitate loans and improve antidote availability.
The establishment of an antidote network can allow to improve the communication between centres managing poisoned patients, as well as to adapt and standar dizethe antidote resources in different centres, and to speed up loans if necessary. Ultimately, it can improve the quality of care for poisoned patients.











 text in
text in