Introduction
Occupational safety has become one of the main concerns in hospitals, and exposure to cytotoxic agents is one of the most relevant issues, as well as accidental needle-sticks. Since the 70s, it has been well known that nurses who prepare chemotherapy presented high levels of mutagenic agents in their bodies1. Therefore, there have been many guidelines and agreement documents2,10 prepared in order to try to minimize the exposure of workers to this type of agents3. One of the first advances in this field was the use of biological safety cabinets in combination with individual protection equipment, and the use of closed systems for preparation and transfer of antineoplastic agents4, according to Royal Decree (RD 665/97) which was subsequently modified (RD 1124/2000 and RD 349/2003)5,6. This Royal Decree regulates closed systems and therefore encourages their use, and sophisticated systems have been launched that will replace the use of traditional needles. These complex devices present heterogeneous costs that require their cost-efficiency assessment7. The Pharmacy Area is legally responsible for this assessment (RD 1591/2009)8, which will allow to select the most efficient devices. In order to conduct this assessment, it will be essential to consider that the increased safety offered for cytostatic handling by these Closed Systems (CS) is obtained at the expense of dead space, higher than that of traditional loading needles, which does not allow the total extraction of the liquid contents from the vials9. Therefore, in this study we have analyzed the potential economic impact of incorporating each one of these systems in a third-level hospital; additionally, a cost-efficiency study has been conducted for said devices, based on the most efficient use of the vials.
Methods
A study was conducted with the objective of measuring the cost-efficiency of some of the CS available in the market. The following CS were assessed:
BD-Phaseal®; Hospira ICU CLAVE® CH 70 and CH74; Baxter-ChemoAI-DE®; Care Fusion Smart Site® and VM04®; Fresenius Extra Spike®; Braun Chemo V Mini Spike®.
These systems include, among other features, an awl that allows to pierce the rubber septum of each vial, remaining affixed through anchorage. They also allow to access the vial contents through a luer connector to the syringe, not requiring the use of needles. All these systems feature 0.2 μm venting filters in all their models, except for BD Phaseal, which features an airtight expansion chamber.
The calculation of the estimated economic impact for one year included:
- The cost associated with the loss of the drug that remains in the system. To this aim, the outcomes from the study were extrapolated with each vial size and CS model included, shown in Table 1 and Table 1cont, to the vials used during one year in our hospital, in the Day Hospital areas for Oncology and Haematology.
Table 1 Classification of the marketed presentations used in Oncology and Haematology Day Hospitals in 2014

Table 1 (cont.). Classification of the marketed presentations used in Oncology and Haematology Day Hospitals in 2014

- The cost of the syringes for cytostatic preparations (attached to the CS for handling during preparation).
- The costs of the CS available at the time of conducting the study (Table 3).
The cost of drug loss was estimated through the weight difference technique. The procedure was conducted as follows:
Empty 10, 20 and 30 ml vials were weighed.
Vials were filled with an innocuous and coloured solution, with the respective volumes (10, 20 and 30 mL).
Each CS model was attached, according to size, to the relevant vial.
Liquid extraction was conducted through each one of the CS.
Each vial was weighed once its liquid contents had been extracted. We applied the weight difference technique regarding the initial weight of the empty vial.
This process was conducted three times by three different operators, with the objective of avoiding any bias caused by the level of skill in the use of these systems: Operator 1: with high experience in cytostatic preparation, conducted in his daily practice, wide knowledge of CS; Operator 2: limited experience, with knowledge of the mechanism and performance, and occasionally handling these preparation materials; and Operator 3, without any experience, with knowledge of the mechanism and performance of CS.
The mean level of the 3 measurements was taken as reference. This specific value for each vial size and CS model was considered as drug loss in ml.
The vials of the marketed presentations of drugs used in Oncology and Haematology were classified into 3 groups, according to their diameter size and volume: 13 mm/10 mL, 20 mm/20 mL and 20 mm/30 mL (Table 1); this allowed to extrapolate the loss of the drug discarded in the vial represented by the use of each CS model, through calculating the cost per drug ml, using the price reported at the date of the study, on February, 2015.
The number of total preparations was obtained from the Oncowin® computer system, and the use of vials from SAP®.
Results
In total, there were 63 tests through difference in weight, with each vial size and CS model measured three times, and the mean level of drug not used in each of the three measurements was used as value of reference. These systems were not available to us; Hospira ICU CLAVE® CH 70, CH74, or Fresenius Extra Spike® for the 10ml vial size. The outcomes are stated in Table 2.
Table 2 Volume (ml) of drug lost during the preparation process
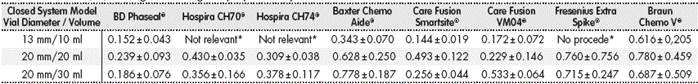
*The vial size is not adequate for using this system.
It was observed that the CS with the best efficient use of the drug in 10ml vials was the system marketed by Care Fusion Smartsite®, with a mean loss of 0.144 ± 0.019 mL, followed by BD-Phaseal® with 0.152 ± 0.433 ml; the system with the highest loss was Braun’s Awl, with 0.661 ± 0.205 mL.
For the 20 mL vial size, the device by Care Fusion® model VM04 allowed the most efficient use of the vial contents (0.229 ± 0.146 mL), followed by BD-Phaseal® with a 0.239 ± 0.093ml loss; the device by Braun® presented the highest recorded loss (0.780 ± 0.459 mL).
For 30 mL vials, the BD-Phaseal® system showed the best results (0.186 ± 0.760 mL), followed by the Care Fusion Smarthsitesystem with 0.256 ± 0.044 mL.
The estimated cost that these losses would represent for the hospital was calculated based on the preparations conducted during 2014 for the Oncology and Haematology Units. In total, 71 different commercial presentations were used, classified by vial size and diameter into 3 groups, as appears in Table 1 and Table 1cont.
During this period, 34,598 cytostatic preparations were conducted in total, using 16,788 vials.
In terms of the different CS models used, the detailed cost was the one available at the time of the study. Table 3 shows the breakdown of these costs by components: chemotherapy, awl, and adapter to the infusion system.
The economic impact represented by the use of each CS model during one year in a third level hospital included the cost of the drug loss, the remains that were not used in each marketed vial, and the cost of CS. The outcomes of this analysis appear in Table 4: we consider that the most efficient CS model would be BD-Phaseal®, with a cost of 255.668,3 €/ year. On the other hand, according to our study, the Braun® model would be considered the least efficient, with an impact of 544,808,9 €/year.
Discussion
Closed Systems have been gradually incorporated in Pharmacy Units, due to the recommendations by our current legislation (RD1591/2009) One of the main drawbacks for their implementation has been their high budgetary impact, as well as the current continuous innovation in this market. The objective of this study was to present an evaluation method for the efficiency of closed systems, through a cost-efficiency analysis, based on the lack of efficient use of the vials shown by these systems.
According to our data, the system with the lowest overall impact on the budget is, however, the one with the highest cost of purchase: the system marketed by BD-Phaseal®; the essential reason for this is that it allows a most efficient use of the drug vials, almost 15% higher than the mean of the other CS.
It is true that this is not the only characteristic that must be assessed at the time of incorporating a new healthcare technology. In fact, this is one of the limitations in our study, which is simply based on cost aspects derived of the purchase of systems and the efficient use of vials. There are even studies that have been published, taking into account the time of preparation of treatments based on the CS used. Besides, currently there are rules that classify these CS into different levels of safety; among these, the BD-Phaseal® systems meet the most demanding requirements. However, we have assumed that all systems present the same sealing and safety data10, and also that there is an equivalent handling of these systems by the nursing staff. The study presented is only a tool for calculating the direct costs derived of the use of different CS. Different hospitals will be able to incorporate these analyses, if they consider them necessary for their assessment of these systems.











 text in
text in 




