Introduction
The natural history of infection by human immunodeficiency virus (HIV) is characterized by a progressive decrease of CD4+ cells and immune function, promoting the occurrence of infections and AIDS -defining malignancies. It is estimated that there are 36.7 million HIV-infected people worldwide and that 1.1 million deaths occur annually from the disease1. In Spain, it affects about 148,785 people2 and its estimated annual mortality is approximately 845 deaths per year3.
Since the introduction of highly active antiretroviral therapy (HAART) as a combination of three antiretroviral drugs, control and maintenance of the di-sease has been achieved in HIV-infected patients and morbidity and mortality have markedly decreased, converting HIV infection into a chronic disease4.
Although Spanish guidelines have been recommending an initial treatment including two nucleoside reverse transcriptase inhibitors (NRTIs) combined with an integrase inhibitor (INI), a non-nucleoside reverse transcriptase inhibi-tor (NNRTI) or a protease inhibitor boosted with ritonavir (PI/r), currently only INI-based regimens are preferentially recommended5.
Fixed-dose combinations of antiretroviral drugs have meant an important step forward in simplifying treatment and improving compliance. Better com-pliance has led to a lower risk of treatment errors and decreased resistance selection, which results in an increased effectiveness of therapy with the con-sequent decrease in viral load and improvement in quality of life of patients6.
Dolutegravir (DTG) is an integrase inhibitor whose clinical development has shown good tolerability and safety, a high barrier to resistance and a lack of relevant drug interactions. The single-tablet formulation with abacavir and lamivudine (DTG/ABC/3TC) obtained European approval in September 2014 and has been marketed in Spain since May 2015.
To assist in the inclusion of the drug on the formulay, the study objecti-ve was to assess the incremental cost-utility ratio (ICUR) in €/QALY of the fixed-dose combination of (DTG/ABC/3TC) versus the combinations Emtrici-tabine/Tenofovir/Efavirenz (FTC/TDF/EFV), and darunavir/r (DRV/r) or Ralte-gravir (RAL) with Emtricitabine/Tenofovir (FTC/TDF) or Abacavir/Lamivudine (ABC/3TC) as initial antiretroviral therapy in patients infected with HIV-1 from the perspective of the Spanish National Health System.
Methods
Description of the model
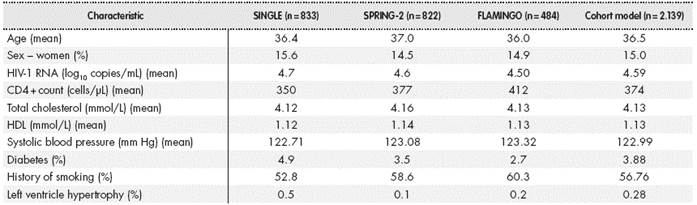
The study consisted of adaptation to Spain of the Anti-Retroviral Analysis by Monte Carlo Individual Simulation (ARAMIS) model7,8, which uses a microsimulation approach to simulate the individual changes in each patient from the start of treatment to death through a chain of descriptive health states of the disease that are mutually exclusive (the patient can only be in one state). Thus, by including the behaviour of each individual generated with baseline characteristics of patients from clinical trials of DTG in naïve patients (SINGLE, FLAMINGO and SPRING-2 trials), the model is able to show the biological variability of the responses that may occur, which is an advantage over typical aggregate approaches such as the Markov models in which the aggregate variables only represent the behaviour of the population mean.
The alternatives used for comparison were the fixed-dose combination of Emtricitabine/Tenofovir/Efavirenz (FTC/TDF/EFV), and the fixed-dose combinations of Emtricitabine/Tenofovir (FTC/TDF) or Abacavir/Lamivudine (ABC/3TC) with Darunavir/r (DRV/r) or Raltegravir (RAL). Thus was obtained the comparing of DTG/ABC/3TC versus standard treatments with NRTIs, INI and a PI/r as a third agent.
As shown in Figure 1, the model defined the following health states: HIV infection without chronic disease as first-line treatment, HIV infection without chronic disease as second- line or subsequent treatment, HIV infection for chronic disease non-related to AIDS (second- line or subsequent treatment), opportunistic disease (viral, bacterial, fungal, protozoan or other), adverse event to the treatment and death (absorbing state).
Each month throughout the simulation and depending on the treatment received, the changes in patient characteristics are determined and if these changes cause the individual to remain in the initial state or change to a new one. These changes are determined by the probability of disease pro-gression, occurrence of opportunistic infections and/or adverse effects, and occurrence of long-term chronic diseases and death. All these probabilities are determined by the CD4+ count the patient has at the start of each cycle.
Thus, depending on the treatment received, the individual will show a given probability of achieving viral suppression (defined as viral load sup-pression below 50 copies/mL at 48 weeks). This leads to an increase in the CD4+ count that is more marked in the first two months of treatment but which is maintained if viral suppression persists, reaching a maximum values of 1,200 cells/µL. If virological suppression is not achieved with treatment initially, or if it is lost subsequently, the individual moves on the next line of treatment and so on. Once treatment options have been exhausted, the individual experiences a decrease in the CD4+ count as described in pre-viously published models9.
The model includes nine categories of adverse events that individuals may experience during treatment (diarrhoea, nausea, dizziness, vomiting, rash, sleep disturbances, insomnia, depression and other). The probability of experiencing these events in grade 2 or higher and of discontinuing treatment due to the events was obtained from their clinical trials.
The occurrence of opportunistic infections was modelled as a probabili-ty dependent on the CD4+ count as described in the literature7.
Cardiovascular disease was modelled as a monthly risk determined by a Framingham equation10 for prediction of coronary heart disease and stroke.
In addition to mortalities due to opportunistic infections and cardiovas-cular diseases, all-cause mortality and HIV mortality were modelled based on Spanish data from interactive consultation of the National Health System Statistical Portal11.
Thus, at the end of each cycle, a specific effectiveness is obtained and the corresponding allocation of costs generated in the period. These are cu-mulative throughout the life of each simulated individual and finally allow for obtaining the incremental cost-effectiveness ratio (ICER) of DTG/ABC/3TC versus other treatment options using the formula:
ICER = ((Cost Triumeq - Cost alternative) / (Effectiveness Triumeq - Effectiveness alternative))
The model was developed in Microsoft Excel 2007 and Microsoft Vi-sual Basic for Applications as embedded code. The results shown corres- pond to 1 million simulated individuals, from the perspective of the Spanish national health system. The base case was performed for a time horizon of the patient’s whole lifetime and applying a discount rate of 3%.
Model parameters
Study population
Initially patients were described with demographic variables (age, sex), disease defining parameters (plasma viral load, CD4+ cell count), and co-variates used for measuring cardiovascular risk in the Framingham equation (systolic pressure, total cholesterol, high- density lipoprotein (HDL) choleste-rol, history of smoking, diabetes and left ventricular hypertrophy).
Table 1 shows a summary of the baseline characteristics used in the model. These were obtained from patients participating in the SINGLE, SPRING-2 and FLAMINGO clinical trials of DTG in treatment-naive patients.
Treatment algorithms
As shown in Figure 2a, Figure 2b, Figure 2c, Figure 2d, an expert panel determined the four treatment algorithms, one for each initial treatment. The choice of treatment in the second and subsequent lines was made attempting to prevent problems of tolerability and resistance that could compromise efficacy. The model took into account resistance to NRTIs, NNRTIs, and INI.
Estimation of effectiveness
The probability of achieving virological suppression by the treatments included in the model was obtained from clinical trials comparing DTG/ ABC/3TC versus the other alternatives, SINGLE trial for FTC/TDF/EFV12,13, SPRING-2 trial14,15 for RAL + (FTC/TDF) or + (ABC/3TC) and FLAMINGO trial16,17 for DRV/r + (FTC/TDF) or + (ABC/3TC). Based on their clinical prac-tice, the expert panel considered that the proportions for applying FTC/ TDF and ABC/3TC in these two alternatives are 80 and 20, respectively. Since there are no direct trials of DTG/ABC/3TC versus Rilpivirine/Emtrici-tabine/Tenofovir (RPV/FTC/TDF) and Emtricitabine/Tenofovir/ Elvitegravir/ Cobicistat (FTC/TDF/EVG/cob), the results used for comparison of these re-gimens were obtained by a network meta-analysis18. The evidence used for showing the long -term efficacy of all treatments was the STARTMRK trial19,20 and assumptions validated by the expert panel.
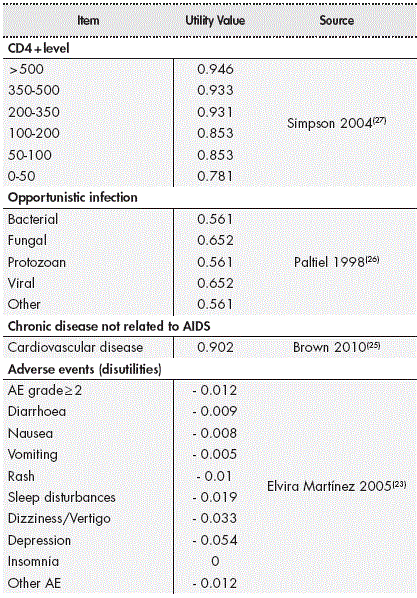
The efficacy of treatment alternatives was measured as life years gained (LYG) and quality-adjusted life years (QALYs) by applying the corresponding utilities associated with CD4+ level, having an opportunistic infection and its sequelae, having an adverse event and those associated with chronic diseases not related to AIDS. These utilities shown in Table 2 were obtained from the literature7,21-24.
While quality of life stratified by CD4 was calculated in each cycle in which the patient was in the health state, in the case of opportunistic infections, it was calculated in three cycles, assuming that this opportunistic infection had a duration of three months. The effect of adverse events was applied in the cycle in which they occurred, except for cases of depression, whose disutility was imputed over a period of three cycles (3 months).
Resources and costs
As the model uses the perspective of the Spanish National Health Sys-tem, only direct health care costs expressed in € (2015) were considered.
Thus, standard patient care costs were considered such as those derived from medical visits, diagnostic tests, management of adverse events, care of chronic diseases and drug costs of antiretroviral therapy and prophylaxis and treatment of opportunistic infections.
Table 3 shows the costs attributable to each event and health state from the official rates and were calculated from the clinical trial protocols and stratified by CD4+ levels22. The costs of diagnostic tests come from the rates of the Instituto de Salud Carlos III. The costs derived from treatment of adverse events for Spain were obtained from the literature25 and the costs derived from the prophylaxis and treatment of opportunistic infections from the BotPlus-Portsalfarma database 2.0 of the General Council of Offi-cial Pharmacists Associations (https:/botplusweb.portalfarma.com)21,26. The drug acquisition costs of the different antiretroviral therapies by the National Health System were obtained from the SESCAM27.
Table 3 Monthly costs

(*) Costs updated to 2015. CMV, cytomegalovirus; PCP, pneumonia by Pneumocystis jiroveci; ABC, abacavir; 3TC, lamivudine.
Sensitivity analysis
In order to assess the consistency of the model and the effect on its results of the relative efficacy of DTG/ABC/3TC versus its alternatives, the time horizon and discount rate applied, as recommended by the proposed Spanish guidelines for economic evaluation28, the following sensitivity analy-ses were performed:
Model using a time horizon of 5 and 10 years.
Application of higher relative and lower efficacy using the maximum and minimum values of the confidence intervals of clinical trials12-17.
Model without application of a discount rate and using a rate of 5%.
Results
Base case
Of all the initial treatment regimens studied, DTG/ABC/3TC was the dominant combination by achieving greater effectiveness at a lower cost (Table 4). The incremental cost-effectiveness plane is shown in Figure 3. Table 5 shows the duration and average monthly cost of each strategy evaluated.
Table 4 Cost-effectiveness results of comparisons analyzed

QALYs, Quality-Adjusted Life Year; ICER, Incremental Cost-Effectiveness Ratio.
Sensitivity analysis
Table 6 presents the results of the comparisons used in sensitivity analy-ses. In these comparisons, DTG/ABC/3TC was the dominant combination (representing a lower cost and greater effectiveness) in all cases versus FTC/TDF/EFV.
Identical behaviour was shown in the comparison with RAL + (FTC/ TDF) or + (ABC/3TC), except in cases of without discounting in which DTG/ ABC/3TC represented a saving of 24,415.86 € for a lower effectiveness of 0.0396 QALYs, and when the lower limit of efficacy was considered for DTG/ABC/3TC, a saving of 19,454.97 € for a lower effectiveness of 0.0511 QALYs. In both cases, the strategy was found to be cost-effective.
Similar results occurred with DRV/r + (FTC/TDF) or + (ABC/3TC), which were shown to be cost - effective in the non-discounted analyses (saving of 9,646.16 € and lower efficacy of 0.036 QALYs) and in the lower limit of efficacy of DTG/ABC/3TC (saving of 8,698.67 € and lower efficacy of 0.049 QALYs). In the rest of the cases, DTG/ABC/3TC was shown to be the dominant alternative.
Discussion
Since the introduction of HAART, control and maintenance of HIV in-fection has been achieved, decreasing its morbidity and reducing costs associated with its medical care. However, the increased survival produced causes costs of treatment and patient care to occur over a much longer time period, normally causing overall costs to become larger than other treatment alternatives and making it necessary to evaluate its cost-effectiveness.
The ARAMIS model used for this adaptation has been verified and pre-viously used in several studies7-8. This is the first research that uses microsimu-lation to assess the cost-utility of a VIH fixed dose combination in Spain. The stochastic approach used in this study enabled us to show the biological variability and to determine that the fixed-dose combination DTG/ABC/3TC is the most efficient alternative among the initial treatment regimens evalua-ted. It was to be the dominant strategy in the comparisons considered as the base case and in 14 of 18 sensitivity analyses, and was cost-effective in the remaining four.
This greater efficiency is determined by the savings derived from starting treatment with DTG/ABC/3TC and because this alternative was the most efficient in all cases, except in the sensitivity analyses without discounting and using the lower efficacy level of the trials with dolutegravir (DTG) when compared to the alternatives RAL and DRV. In these cases, the lower effica-cy of the alternative to starting with DTG/ABC/3TC was shown to be mar-ginal with values between -0.04 and 0.03 QALYs, which over the lifetime of the patient are equivalent to a lifetime benefit of between 10 days and two weeks. This marginality of the unprovided benefit causes the savings produced to make the alternative appear cost-effective with respect to the compared alternatives.
This effectiveness is consistent with the proposed treatment algorithms since all alternatives are favoured by having treatments including DTG in subsequent lines. Thus, the effectiveness shown in the first lines of treatments is consistent with the efficacy results of the comparative clinical trials of the treatments evaluated12-17. And this dominance is consistent with the results of previous research compararing DTG with ABC/3TC or FTC/ TDF in Canada29.
As shown in Table 5, this efficiency of the first lines of the alternative to starting with DTG/ABC/3TC is boosted by a lower mean monthly cost of treatment than the other compared strategies. Furthermore, it can be seen how other alternatives are benefited by the use of DTG/ABC/3TC in the second lines of treatment.
Although the model has limitations due to the lack of data on the efficacy of subsequent treatment lines and the costs derived from visits in actual clinical practice, these have been treated conservatively. Thus, the high efficacy applied to treatments after the first failure will mean that patients continue with high CD4+ levels. Hence, the benefit of postponing the change of initial treatment shown in the alternative to starting with DTG/ABC/3TC is masked by the effect of subsequent lines. With regard to the case to imputing only protocol visits, this is also a consequence of a conservative approach because it does not consider unexpected visits occurring in actual clinical practice as a result of adverse effects and interactions, regardless of whether or not it involves discontinuation. It is considered that this assumption could penalize the strategy of starting with DTG/ABC/3TC since it safety profile, tolerability and interactions suggests that this treatment generates a lower number of these visits, which leads to underestimating the costs attributable to the other compared strategies. Fi-nally, another limitation has been that the study has not taken into account the subsequent introduction of the generic ABC/3TC
Cost-utility analyses of these alternatives based on real World data of their clinical effectiveness and associated resource consumption will be recommended in the future.
Since the results obtained with this model are favourable to the strate-gy of starting with DTG/ABC/3TC, it may be concluded that, this is the most efficient option of the alternatives evaluated for the Spanish National Health System. This result could assist informed decision-making about inclusion of the fixed-dose combination DTG/ABC/3TC into the hospital formularies.