Introduction
Infiltration of neoplastic cells into the central nervous system (CNS) is a rare complication in patients with acute leukaemias and non-Hodgkin’s lymphoma (NHL), but when it does occur, it is associated with a high morbidity and mortality rate1.
The use of prophylaxis or neuromeningeal infiltration treatment is normally included in the treatment protocols and, in general, the administration of intrathecal chemotherapy is recommended. However, therapeutic suitability, the standard used and the drugs and doses to be administered in intrathecal therapy are not applied uniformly amongst countries and/or working groups, or even amongst different hospitals. In Spain, according to the QUIT survey, the combined administration of methotrexate, cytarabine and hydrocortisone, known as triple intrathecal (TIT) chemotherapy, is used in most cases2-3.
In addition to this non-uniform application, there are few studies that evaluate tolerance of intrathecal chemotherapy administration. Furthermore, other aspects that, according to several authors, may affect toxicity, such as the volume, pH and osmolarity of the solution, as well as aspects of the administration technique, are not normally indicated in the existing studies and could affect the results4,5.
Therefore, an internal hospital protocol was drafted to standardise preparation and provide recommendations for TIT administration.
The purpose of this study is to evaluate the toxicity associated with standardised administration of triple intrathecal chemotherapy and to identify associated risk factors.
Methods
A prospective observational study was conducted on all standardised TIT administrations given to adult haematology- oncology patients (age ≥ 18 years) over an 18-month period (January 2013 - June 2014). Patient follow-up was conducted until 31 July 2014.
Triple Intrathecal Chemotherapy
The methotrexate, cytarabine and hydrocortisone doses were extracted from the PETHEMA (Programa para el Estudio y Tratamiento de las Hemopatias Malignas, Programme for the Study and Treatment of Malignant Blood Disorders) protocols, at 12 mg, 30 mg and 20 mg, respectively. The final volume of the solution was 8 mL. The preparations were adjusted to pH and osmolarity values similar to those of cerebrospinal fluid (CSF), using 0.9% sodium chloride as a solvent (osmolarity approximately 300 mOsm/L) and adjusting the pH to 7-7.5 with sodium bicarbonate6,7.
The preparations were sterile, non-pyrogenic and free of preservatives.
TIT Administration
Recommendations were established in order to improve tolerance: (1) extract a volume of CSF similar to the volume of TIT to be administered; (2) perform lumbar puncture (LP) with the patient seated or in the lateral decubitus position during administration; (3) use local anaesthetics prior to administration; (4) have the patient rest 2 hours in the supine position following administration.
Variables studied and statistical analysis
Adverse events (AE) occurring after administration of TIT chemotherapy were recorded, as well as several patient- and administration-related variables that could have influenced toxicity. Adverse effects were defined and evaluated based on the CTCAE v4.0 classification8, and causality was studied using the Naranjo Scale9.
The relationship between the variables and the presence or absence of toxicity was analysed using the Chi-Square test (qualitative or categorical variables) or the Student-t or Mann-Whitney U tests (quantitative variables). A logistic regression analysis was conducted with toxicity as a dependent variable and variables in which a relationship with toxicity had been observed as covariates. Variables with p-values < 0.15 in univariate analysis were included in the multivariate regression model. A p-value of p < 0.05 was considered significant.
Results
A total of 56 standardised TIT administrations were recorded in 20 patients, with a mean age of 47.7 ± 13.8 years (Table 1).
Table 1 Clinical characteristics and demographics of the patients included
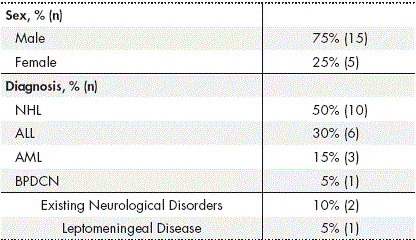
*Abbreviations: ALL: Acute Lymphoblastic Leukaemia; AML: Acute Myeloid Leukaemia; NHL: Non-Hodgkin Lymphoma; BPDCN Blastic Plasmacytoid Dendritic Cell Neoplasm.
Amongst all administrations, 94.6% were given as prophylaxis for CNS infiltration and only 3 procedures were performed as treatment, all in the same patient. The median number of TITs administered prior to the recorded TIT was 2 (interquartile range (IQR) = 0.25-7). The median time interval between administrations was 28 days (IQR = 20.5-51 days).
Prior cranio-spinal radiotherapy had not been administered in any of the patients, although total body irradiation (TBI) had been administered in 2 procedures (1 patient) and concomitant TBI had been administered in 10 procedures (17.9%) (5 patients). In 76.8% of the administrations, the patient was receiving concomitant chemotherapy, and in 75% the patient received a potentially neurotoxic antineoplastic drug.
All procedures were conducted by means of lumbar puncture. In 62.5% of administrations, the patient remained in a lateral decubitus positionduring the procedure, and following the procedure, 94.6% remained in a supine position. Local anaesthetics were used prior to administration in 19.6% of administrations. The post-puncture resting time was recorded in 24 administrations (42.8%), with a median time of 1 hour (IQR = 1-2h) and a maximum resting time of 4 hours. Complications arose during the procedure in just 2 cases; both were minor and did not require treatment.
The volume of TIT administered was 8 mL in all procedures, and the mean difference between the volume of drug administered and CSF extracted was 2.7 ± 2.2 mL (Range = 1-6.75 mL).
In 87.5% of the procedures the patient was hospitalised; the rest of the administrations were performed on an outpatient basis. The in-hospital observation time was 13 ± 11 days for inpatient care and 2.8 ± 0.7 hours for outpatient care.
AEs were recorded in 39.3% of the administrations (22), affecting 70% of the patients included in the study. In 95.5% of the administrations in which AEs were detected, the patients were receiving inpatient care. In 22.7% of the administrations in which AEs were detected, the patient was receiving concomitant TBI; in 86.4%, chemotherapy; and in 68.2%, a potentially neurotoxic antineoplastic drug.
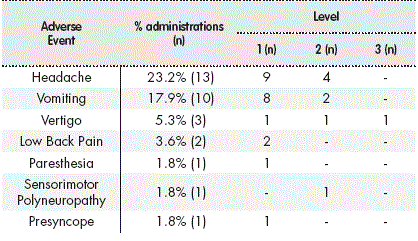
In 8 procedures, more than one AE was detected (2 AEs in 7 procedures and 3 AEs in 1), with a total number of 31 AEs recorded in 7 clinical events (Table 2). The severity in 96.7% of the cases was minor to moderate; only one adverse event was level 3. Symptomatic treatment was required in 77.4% of the cases.
The median time to event onset following TIT administration was 20 hours (IQR = 4-48h). The median duration of the AEs was 48 hours (IQR = 5-144h).
Applying the Naranjo Scale, a probable causal relationship was established between the TIT and the AE in 6.5% of the AEs, with a possible relationship in 77.4% and an uncertain relationship in 16.1% of AEs.
When comparing the variables collected during the study, between TIT administrations in which toxicity was and was not detected, statistically significant differences were observed in (median (interquartile range)): patient age (43 (31.5-53.7) vs. 52.5 (41.2-59.2) years, p = 0.019), days between administrations (20 (4-32) vs. 31 (22-74), p = 0.010), hospital observation time (360 (90-600) vs. 84 (3-462) hours, p = 0.042) and in the difference in volume of CSF extracted and drug administered (3.2 (2.7 -5) vs. 3 (0-3.6) ml, p = 0.036). No statistically significant differences were observed in: appearance of complications during the procedure, inpatient vs. outpatient care, purpose of the TIT (prophylaxis or treatment), prior or concomitant CNS irradiation, concomitant chemotherapy, concomitant neurotoxic drugs or number of prior TITs. The results of the logistic regression analysis for the variables associated with toxicity are shown in Table 3.
Discussion
The administration of TIT chemotherapy, under the controlled conditions described, has proven to be a relatively safe procedure, with a single episode of level 3 toxicity; the rest of the adverse events recorded were of minor to moderate severity, and all cases were acute or subacute and self-limiting, requiring only symptomatic treatment. It is worth noting that in 86.4% of all administrations with an AE, the patient was receiving concomitant chemotherapy or radiotherapy, and in 68.2% the patient also received some type of neurotoxic drug, which makes it considerably complicated to determine whether these AEs were due to the TIT treatment or to the systemic treatment of haematological malignancies. Furthermore, most of the AEs recorded could be due to complications associated with the administration procedure itself, as they have been described as complications of the LP: post-puncture headache, low back pain, nerve root irritation, subdural haemorrhage, inter alia10. Due to this complexity in establishing a causal relationship between AEs and TIT, when using the Naranjo Scale, the relationship was considered possible in most cases and definite in none of them.
There are few published studies that describe toxicity due to TIT chemotherapy in adults11-16. Furthermore, these studies vary greatly in their methodologies, making it extremely difficult to compare the results of the present study directly with those reported by other authors. Hitchins et al.11, prospectively, and Kim et al.12, retrospectively, focus their studies on patients with meningeal carcinomatosis due to solid tumours; neither describes the appearance of severe neurotoxicity. The studies by Huguet et al.13, Thomas et al.14 and Storring et al.15 use TIT chemotherapy as part of the treatment protocol for ALL in adults; only the study by Storring et al. describes the appearance of level 1-2 headaches and nausea as a result of CNS prophylaxis, although no incidence data was provided15.
Pardo et al.16 retrospectively analysed toxicity associated with intrathecal administration of different drugs (TIT, methotrexate, cytarabine, trastuzumab and liposomal cytarabine) in a total of 627 procedures; an AE was recorded in 9.4% of cases. Excluding the administration of liposomal cytarabine, which is associated with greater toxicity, only 8% of the remaining 537 procedures (80% TIT) recorded an AE. However, unlike our study, 40.9% of them were considered severe. The adverse effect recorded most often by Pardo, as in our study, was headache, possibly attributed to the intrathecal chemotherapy, according to the Naranjo Scale. However, this author does not include emesis as an AE possibly related to IT administration, which may contribute to the difference in the results.
None of the studies cited describe the conditions for conducting the procedure, the volume of IT solution administered and whether a similar volume was extracted, or the method of preparation and final condition of the solution. Nor do they tend to indicate the use of premedication, position during the puncture or the amount of post-puncture rest, aspects that are indicated in this study asadditional information. Of those factors, the only one of note is that the amount of rest was less than the recommended time.
Good TIT chemotherapy tolerance by the patients in our study seems to suggest that the TIT solution preparation and administration conditions are suited to achieving a good safety profile.
In a paediatric population, with the same methodology, toxicity was observed less frequently (16.7% of the procedures), although with a similar profile; most of the AEs detected were level 1-2, with vomiting, headache and low back pain the most frequent17.
As regards the relationship between toxicity and the study variables, only in the multivariate analysis did we observe a relationship to the age of the patient and the difference in volume extracted and administered.
Age acted as a protective factor in the appearance of toxicity, with a 0.95 risk that a patient of a certain age of will suffer an AE compared to that of a patient one year younger. Although a greater risk of toxicity has been described in older patients due to systemic chemotherapy, especially neurotoxicity with high doses of cytarabine, there are no data in the literature associating age in adult patients with toxicity due to IT chemotherapy. The relationship observed between lower age and toxicity may not be due specifically or exclusively to age, but rather to the fact that a more aggressive chemotherapy regimen tends to be used in younger patients, and systemic chemotherapy could influence the appearance of the toxicity described. Furthermore, the risk of emesis is greater in younger patients, and this adverse effect was included in our study due to its possible association with the IT treatment.
A greater difference between the CSF extracted and the volume administered acted as a risk factor in the development of toxicity. The appearance of headache, nausea, vomiting and obtundation, due to increased intracranial pressure when administering a volume of drugs greater than the CSF extracted, is described in the literature4,16. However, despite the results obtained, it is not probable that an increase in intracranial pressure would occur due to a volume increase of 1 to 6.75 mL, taking into account that the volume of CSF in an adult is between 125 and 150 mL. Therefore, the underlying mechanism for this toxicity must be influenced by other factors, such as the rate at which the additional volume is administered15.
As regards limitations, note that the patient follow-up time may have been insufficient to detect the appearance of some adverse reactions related to IT chemotherapy in the long term. The 12.5% of outpatient administrations may have led to a loss of information, with a subsequent underestimated frequency of toxicity, especially in mild symptoms. Furthermore, the high level of complexity in the sample patients and the occasional difficulty in differentiating between toxicity due to the drug and to the procedure itself has made it difficult to establish a causal relationship between toxicity and IT chemotherapy.
Administration of triple intrathecal chemotherapy under controlled conditions has proven to have a good safety profile. The most frequent adverse events were minor-to-moderate intensity headache and vomiting. Risk factors for toxicity that were identified included lower patient age and greater difference between the volume of CSF extracted and that of the drugs administered.
Contribution to the scientific literatura
To the best of our knowledge, this study is the first prospective study focusing on the evaluation of toxicity in the administration of intrathecal chemotherapy in a controlled setting in adult haematology-oncology patients. This study describes the use of standardised solutions of methotrexate, cytarabine and hydrocortisone in saline solution, with pH and osmolarity adjusted to the physiological range of cerebrospinal fluid. This description of the standardised solution, together with that of the controlled administration conditions, may be of great use to various professionals as, although the administration of intrathecal chemotherapy is widespread, there is very little pertinent information in the literature on these aspects.
It is important to highlight that this study evaluates real health results. It describes a protocol for using intrathecal chemotherapy that provides a good toxicity profile. It is also important to highlight the study of predictive toxicity factors that may help foresee the risk of toxicity in different populations.











 texto en
texto en