Introduction
Cystinosis is a rare lysosomal storage disorder that follows an autosomal recessive inheritance pattern1. This metabolic disorder is characterized by the accumulation of the amino acid cystine in lysosomes due to defective cystine transportation from the interior to the exterior of the lysosome2,3. Cystine has low solubility in water, leading to the formation of intralysosomal crystals and damage to various tissues and organs, including the cornea. As described by Burki in the 1940s, the ocular manifestations of the disease are due to the accumulation of cystine crystals in the ocular surface4. These crystals can be observed with a slit lamp and are a pathognomonic sign of cystinosis. They begin to form during infancy and from 16 months of age onward they can be observed through a slit lamp. Patients are initially asymptomatic. Due to the accumulation of corneal cystine crystals over time, ocular symptoms do not appear until approximately 10 years of age5.
The specific treatment of cystinosis is cysteamine, also called mercaptamine or 2-aminoethanethiol, which is an aminothiol with chemical formula HSCH2CH2NH26. Cysteamine was introduced as a possible therapeutic agent for cystinosis in 1976 and remains the only available treatment7. Although cysteamine does not cure cystinosis, it has revolutionized patient management and prognosis. It has been shown to slow disease progression and can reduce the amount of intracellular cystine by more than 90%. Cysteamine therapy should be started as soon as the diagnosis is made and should be continued for the lifetime of the patient. Patients with poor adherence to treatment or begin it later do not achieve such beneficial outcomes8.
Oral cysteamine is administered in the form of cysteamine bitartrate, but does not reach the cornea due to the lack of corneal vascularization. Thus, a topical ocular application was developed, whose safety and effectiveness had already been demonstrated in the 1980s9-11. Currently, there are two available ophthalmic formulations of cysteamine hydrochloride: Cystaran® (Sigma Tau Pharmaceuticals Inc.), an FDA-approved medication, which must be instilled from 6 to 12 times a day12; and Cystadrops® (Orphan Europe, Paris, France), which has a higher viscosity and increased ocular permanence13,14. Cystadrops is currently in Phase III trials; however, the European Medicines Agency has recently allowed it be marketed as an orphan drug to facilitate access15.
Access to foreign and/or orphan drugs can sometimes be delayed by the obligatory procedures and approvals required for their use. In addition, the sometimes exorbitant price of these drugs can hamper access16. In order to facilitate the treatment of ocular cystinosis, cysteamine eye drops as a compounded formulation are commonly prepared in hospital pharmacy services.
Two major problems are associated with these formulations. Firstly, cysteamine eye drops must be instilled every hour while the patient is awake to reduce the amount of corneal crystals. To optimise the formulation and avoid these difficult dosage schedules, our group developed a bioadhesive cysteamine hydrogel with high ocular permanence, which could be prepared by hospital pharmacy services17,18. Secondly, there is a lack of studies on the stability of cysteamine formulations. The analysis of compounds with thiol groups has always proved difficult, owing to their susceptibility to oxidisation and the lack of a structural chromophore needed for their detection19,20. Furthermore, the low molecular weight of cysteamine (MW = 77.15 g/mol) hinders its direct detection by mass detectors. Thus, the methods used to determine these types of compounds usually derivatize the cysteamine molecule before quantification21.
The objective of this article was to determine the stability of a bio-adhesive ophthalmic cysteamine hydrogel under different storage conditions.
Methods
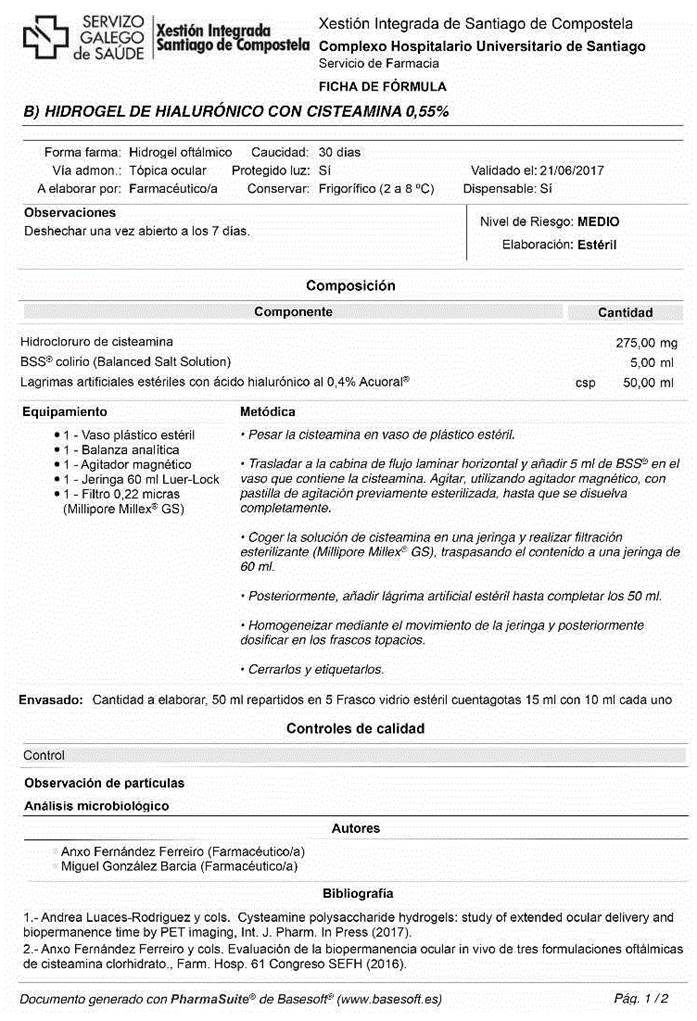
Preparation of 0.55% ophthalmic cysteamine hydrogel
The preparation of the hydrogel is performed in 2 stages. Firstly, a sufficient quantity of cysteamine (BioXtra, Sigma-Aldrich) is gradually added to Balanced Salt Solution Alcon® and magnetically stirred over a period of 5 minutes to achieve a concentration of 0.55%. While continuing to stir, hyaluronic acid (Acofarma®) is then added to achieve a final concentration of 0.4%.
Secondly, the resulting hydrogel is vacuum filtered using a 0.22-μm membrane filter (Stericup® Merck Millipore ExpressTM PLUS 0.22 μm) and poured into 15-mL type -1 amber glass containers, adding 10 mL of hydrogel to each container. The remaining volume is filled with nitrogen gas, and the containers are closed. The entire process is performed under aseptic conditions using a horizontal laminar flow hood.
Preservation conditions and study variables
The formulas were divided into 2 batches: those without preservatives and those with preservatives. The latter batches were prepared by adding 0.01% Ethylene Diamine Tetraacetic Acid (EDTA) while dissolving the cysteamine. Half of the batches with and without preservatives were stored for 30 days at 22 ºC (room temperature) and the other batches were stored at 4 ºC (refrigerated). In the rest of this article, these formulations are referred to as HA (room temperature without EDTA), HAE (room temperature with EDTA), HN (refrigerated without EDTA), and HNE (refrigerated with EDTA).
Osmolality, pH, and cysteamine concentrations were assessed. Descriptive tests were based on transparency measurements, and microbiological tests were based on sterility testing.
All samples were allowed to reach and stay at room temperature for a minimum of 30 minutes to avoid measurement errors due to temperature variations. All tests were performed in triplicate and were conducted on days 0, 7, 14, and 30 after the preparation of the hydrogels.
Descriptive tests
The transparency of the samples was determined by measuring transmittance in the visible light range (380 nm - 780 nm) using a UV-VIS spectrophotometer (model 8452 Diode Array Spectrophotometer, Hewlett Packard). A blank was made with distilled water, the different formulations were placed in quartz cuvettes, and then transmittance was measured, thus obtaining a graph representing the percentage of transmitted light as a function of wavelength.
Physicochemical tests
Determination of osmolality and pH: Osmolality was measured using a vapour pressure osmometer (VAPRO 5520). 10 μL of each formulation was deposited on a disk of Whatman filter paper on the chamber. pH was determined using a Crison micropH2001® pH-metre.
Quantification of cysteamine: A saturated solution of Ellman’s reagent (5,5’-dithiobis -(2- nitrobenzoic acid)) was prepared as a derivatizing agent 22 (Figure 1), by dissolving 29.6 mg of the powder in 10 mL of 0.018 M aqueous NaOH. Subsequently, the solution was filtered through a 0.45 μm filter.
To quantify cysteamine, a 1:1000 dilution of the formulation was prepared. 100 μL of Ellman’s reagent and 100 μL of purified water were added to this diluted sample. The resulting solution was analysed using an ultra-performance liquid chromatography-tandem mass spectrometer (UPLC-MS/MS) method. Measurements were taken using an Acquity UPLC® H-Class system (Waters ® Milford, Massachusetts) coupled to a Xevo TQD mass spectrometer (Waters®). Data were collected using Mass lynx v4.1 software and processed using Target LynxTM Application Manager chromatographic software. Chromatographic separation was conducted at 40 ºC using an Acquity BEH C18 column (2.1 mm x 50 mm; particle size 1.7 µm) (Waters®). The mobile phase solvents used were a 0.1% formic acid solution in water (MilliQ®) (Phase A) and acetonitrile (Phase B). A gradient with constant flow rate of 0.4 mL/min was used. The gradient was started at 100% phase A, changing linearly to a 40% A - 60% B composition at 2.2 minutes, maintaining the composition until the 2.60-minute mark, and then returned to initial conditions at 3 minutes. The autosampler was set to 10 ºC and 10 μL of each sample was injected. Total run time was 3 minutes, which included equilibration of the chromatographic system prior to sample injection. Mass spectrometry data were obtained using the multiple reaction monitoring (MRM) mode through positive electrospray ionization. Quantification was achieved by means of the transitions of the precursor ion at m/z 313 and the 196.85 fragment ion using a desolvation gas flow rate of 1.1 L/h, cone gas flow rate of 80 L/h, and a capillary voltage of 3.2 kV. Desolvation and source temperatures were 600 ºC and 146 ºC, respectively.
Microbiological tests
Each of the hydrogels was analysed on the aforementioned days to determine microbiological stability. 1 mL of each of the hydrogels was added to plates containing blood agar, sabouraud agar, and fluid thioglycolate medium. The samples were cultured at 37 ºC for 48 hours, 15 days, and 10 days, respectively. At the end of each incubation period, the samples were inspected for any signs of microbiological growth.
Allowed variation range and statistical analysis
The Pharmaceutical Codex was used to establish the expiry date of the formulation, which was set23 when there was a 10% reduction of active ingredients compared to the initial concentration. Changes in pH and osmolality were considered unacceptable if their values exceeded the acceptance criteria for ophthalmic applications. Microbiological stability was considered acceptable providing no microbial growth was detected in the cultured samples. Finally, the product was considered unacceptable in the absence of complete transparency on descriptive tests.
The results of the different preservation conditions were compared by multivariate analysis of variance using Graph Pad Prism® v.5.0b software.
Results
Descriptive and physicochemical tests
All the formulations were completely transparent and no decrease in transparency was observed over the study period. No signal was observed in the visible range, demonstrating the transparency of the sample (see Figure 2).

Figure 2 Graph obtained by determining the transparency of one of the formulations, showing negligible absorbance in the visible light spectrum (380 nm - 780 nm).
Figure 3 shows variations in osmolality of the cysteamine hydrogel under all four preservation conditions over time. Osmolality values of all formulations remained between 90% and 100% of the initial values over the study period. Under the different study conditions, no statistically significant differences were observed between the formulations, although those containing EDTA showed slightly higher values (427 ± 8.96 mOsm/Kg vs 410 ± 9.48 mOsm/Kg).

Figure 3 Percentage change in osmolality (mOsm/Kg) (mean and standard deviation) of cysteamine hydrogel over 30 days under the four different storage conditions. HA, room temperature without EDTA; HAE, room temperature with EDTA; HN, refrigerated without EDTA; HNE, refrigerated with EDTA.
However, as shown in Figure 4, neither the addition of EDTA to the hydrogel nor storage temperature influenced the pH values of the hydrogel over the study period. Under all the conditions tested, no statistically significant differences were observed between the initial and final pH, except for a slight decrease in pH in the EDTA formulations (6.29 vs 6.44).

Figure 4 Change in pH (mean and standard deviation) of cysteamine hydrogel over 30 days under the four different storage conditions. HA, room temperature without EDTA; HAE, room temperature with EDTA; HN, refrigerated without EDTA; HNE, refrigerated with EDTA.
Concentration of cysteamine
A narrow, symmetrical, and well-defined chromatographic peak was obtained with an elution time of 0.33 minutes. Figure 5 shows an example chromatogram of the derivatised cysteamine obtained using the UPLC-MS/ MS method.

Figure 5 Example of chromatogram obtained for cysteamine derivatized with Ellman´s reagent, using the UPLC MS/MS method.
The UPLC-MS/MS determination method employed is highly specific, because it combines the efficiency of chromatographic separation and the high selectivity of a tandem mass detector to select the chemical structure to be determined. Using this method, the derivatised product was separated from any compounds that may have formed from cysteamine degradation.
Figure 6 shows variations in cysteamine concentrations over time under the four different storage conditions. Cysteamine concentrations did not fall below 90% at any point during the study period. Nevertheless, it should be noted that a wide range of concentration values was observed at different time points during the storage period. Some samples had percentages of more than 100% of the initial concentration. This variability was caused by the high viscosity of hydrogels, which makes it difficult to obtain reproducible volumetric samples by aspiration.

Figure 6 Percentage of change in cysteamine concentrations over 30 days. HA, room temperature without EDTA; HAE, room temperature with EDTA; HN, refrigerated without EDTA; HNE, refrigerated with EDTA.
Microbiological stability
Adequate storage of samples was maintained under all study conditions, and no microbial growth was observed in any of the hydrogels during the storage period.
Discussion
Difficult dosage schedules are the major challenge to the use of ophthalmic compounded formulations of cysteamine hydrochloride, because they require hourly instillations to reduce the amount of corneal crystals. A preclinical study has shown that the biopermanence of the hydrogel under study is similar to that of Cystadrops®(18. Quantitative PET biopermanence studies have shown that cysteamine hydrogel with hyaluronic acid has a 60-minute half-life, which is much higher than the 18-minute half-life of cysteamine eye drops typically prepared by hospital pharmacy services. In addition, more cysteamine reaches the stroma after administration of this hydrogel than reaches it with eye drops, and there are statistically significant differences in transcorneal permeation values between the two media. This hydrogel formulation achieves a controlled release of cysteamine over time, and can be prepared by pharmacy services for patients with ocular cystinosis17. Two preparation methods are presented in the supplementary materials.
Stability studies are a relevant technical and economic challenge for hospital pharmacy services and are becoming more frequent to guarantee the quality of the prepared drugs24. The present study investigated the stability of cysteamine hydrogel with hyaluronic acid, and showed that its properties were unchanged in a variety of storage conditions over the study period. Other authors have shown that at room temperature cysteamine oxidises into its cystamine dimer, which is ineffective for the removal of corneal cystine crystals25. For this reason, nitrogen was used to remove environmental oxygen before sealing the containers.
The choice of EDTA as a preservative was based on previous publications, which have shown it to be the most suitable preservative for use in cysteamine formulations. The lowest possible concentration of EDTA was chosen to minimize potential toxicity on the corneal epithelium20,26-28. Other preservatives, such as benzalkonium chloride, were ruled out after an unfavourable benefit-risk assessment by ophthalmologists. This decision was based on their potential epithelial toxicity, which would be higher during the chronic use of the hydrogel under study29.
According to the United States Pharmacopoeia, the stability of a compounded formulation is defined as the amount of time during which a product maintains, within very specific limits, the properties and characteristics that it possessed at the time of manufacture, throughout storage, and during use30. Stability studies determine how the quality of a drug varies over time under the influence of a number of factors, and use this information to provide recommendations on its expiration date and storage conditions. Over the study period, the pH and osmolality of the hydrogels remained practically constant, with no statistically significant differences (α <0.001) between the initial and final values under all storage conditions. The addition of EDTA to the formulation led to a slight increase in osmolality and a decrease in pH without affecting the stability of cysteamine. All the measures applied showed that transparency was 100%. Cysteamine hydrogel with hyaluronic acid maintained its properties for 30 days after preparation. However, because the addition of EDTA did not improve stability and the use of benzalkonium chloride as a preservative was discarded, it is recommended that the hydrogel should be stored in a refrigerator to prevent microbiological growth and as an alternative method to sealing the containers with nitrogen31.
The properties of cysteamine hydrogel have been described in previous studies and the results of this stability study show that the use of cysteamine hydrogel may increase therapeutic benefit in patients with ocular cystinosis. In addition, this formulation may be an effective alternative to those not marketed in Spain, but which are available for importation. The cost of imported formulations is €37,728/y/patient, whereas the estimated cost of producing the formulation is €1,080/y/patient.
The authors conducted a survey using the mailing list of the Spanish Society of Hospital Pharmacy (SEFH) and estimated that there are currently 39 patients under treatment with ocular topical cysteamine in Spain. Thus, the cost of this drug acquired through the Access to Medicines not Authorized in Spain process would be €1,471,392/y. The use of cysteamine hydrogel prepared in hospital pharmacy services would provide patients with better access to treatment and achieve significant savings for the Spanish National Health System.
Contribution to scientific literatura
Cysteamine eye drops are commonly prepared as a compounded formulation in hospital pharmacy services. Two problems are associated with these formulations: their low permanence at the ocular level and the lack of studies on their stability. This article is the first study on the stability of a cysteamine hydrogel with high ocular permanence, which could be prepared by hospital pharmacy services, and may therefore represent a great advance in the treatment of ocular cystinosis.











 texto en
texto en