Introduction
Ovarian cancer (OC) was the fifth most frequently diagnosed cancer in women in Spain in 2015 with 3,228 new cases, ranking sixth in terms of 5-year prevalence with 7,925 cases in 20121. In spite of continuous advances in the identification of hereditary OC, surgery and the introduction of new therapies2, OC mortality remains considerable. In 2012, OC was, after breast cancer (15.5%)1, the sixth leading cause of cancer death in women and the second cause of gynaecological cancer in Spain. The high mortality associated to OC may be explained, at least in part, by the non- specific clinical presentation, which makes an early diagnosis difficult and means that approximately 75% of patients are diagnosed at advanced stages3, leading to a worse prognosis, with a 5-year survival rate of 18.6% for stage IV4.
The majority of histological types of OC are of epithelial origin (~90%)5 and, of these, high-grade serous ovarian cancer (HGSOC) is the most common, accounting for 70% of cases6. Additionally, it is characterized by a potential alteration in the BRCA1 and BRCA2 oncogenes in approximately 20% of cases7.
Each histological and/or molecular subtype is associated with distinct clinical behaviour, but historically they have been treated as a single entity. The combination of surgery and platinum-based chemotherapy is the gold standard for the first-line treatment2 of patients at advanced stages. However, 70% of patients relapse at 3 years5, resulting in multiple treatment lines. Most patients respond to platinum therapy and are considered platinum-sensitive when there is a progression-free period of > 6 months from the last dose2. Standard treatment may be combined with anti-angiogenic therapy administered as maintenance monotherapy until progression, as it has been suggested that maintenance therapies administered to patients with a partial or complete response to chemotherapy may delay or even prevent recurrences8.
Olaparib has recently been approved as maintenance monotherapy in adult patients with BRCA mutation positive, platinum-sensitive (complete or partial) recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. Olaparib is a poly ADP ribose polymerase inhibitor (PARPi), an enzyme involved in the repair of damaged DNA. It is the first approved PARPi and the only oral maintenance therapy in this subgroup of patients, and has been shown to delay disease progression9, although its economic impact for the Spanish health system has not been estimated yet.
The objective of this study was to estimate the economic impact of the introduction of olaparib in the Spanish National Health System (SNS) as maintenance monotherapy in adult patients with BRCA-mutation positive HGSOC across different treatment lines.
Methods
A model was developed in Microsoft Excel 2013 with the different therapeutic sequences recommended through which a patient cohort moves across four treatment lines. The perspective of the analysis was the National Health System (NHS) and time horizon was 5 years. Two clinically equivalent cohorts of patients were compared in the two scenarios: with and without olaparib.
Target population
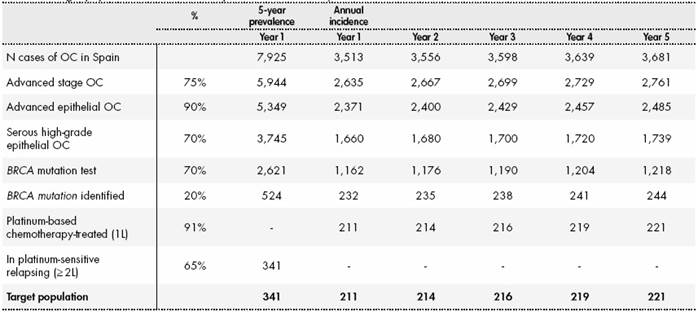
The model included prevalent and incident patients diagnosed with BRCA-mutation positive, platinum-sensitive (PSR) HGSOC according to the indication for olaparib. The annual incidence and the prevalence of OC in Spain were obtained from the GLOBOCAN database1.Prevalent patients enter the model in first year of the analysis from the second line of treatment onwards, while incident patients enter the model each year in the first line of treatment.
The base case scenario considered that 75% of patients would have advanced OC3, with 90% having epithelial histology5 and 70% high-grade serous cancer6. It was assumed that 70% of patients undergo BRCA1/2 gene testing, with 20% being positive for a deleterious mutation in one of these genes7. Finally, it was considered that 91% of incident patients would start first line treatment with platinum-based chemotherapy, and that 65% of prevalent patients had previously been treated with at least one line of chemotherapy to which they responded10. The 341 estimated prevalent patients in the first year of the analysis were distributed between treatment lines as follows: 27%, 34%, 22% and 17% for the 2nd, 3rd, 4th and > 4th lines, respectively (data on file). All these calculations for target population are shown in (Table S1) (Supplementary Material).
Treatment alternatives
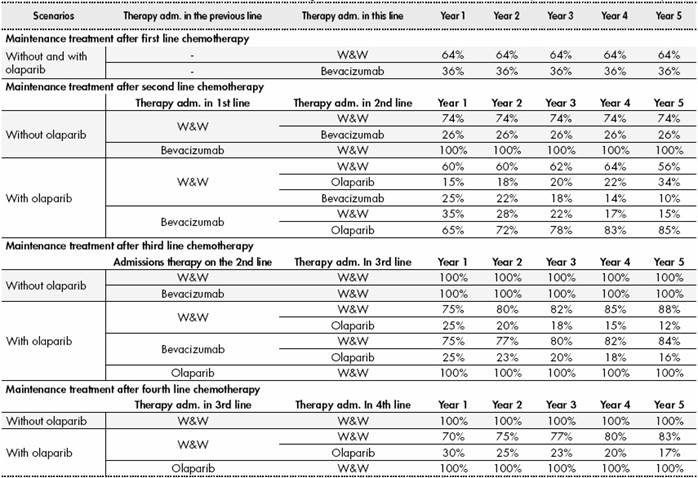
Across all treatment lines, patients received a 6-cycle platinum-based chemotherapy regimen consisting of the combination of carboplatin plus paclitaxel, gemcitabine or pegylated liposomal doxorubicin (PLD) following the recommendations of guidelines2 and the indications of the summary of product characteristics. Subsequently, patients received maintenance treatment until disease progression, consisting in: either watch and wait (W&W), bevacizumab or olaparib (Figure 1).The assumed distribution of use according to market data (data on file) of each doublet chemotherapy and maintenance therapy is shown in (Table S2) and (Table S3) (Supplementary Material).
Table S2 Distribution of platinum-based chemotherapy regimens among the patients through the four treatment lines for olaparib and W&W as maintenance therapy*

* For bevacizumab as maintenance therapy, Carboplatin + Paclitaxel and Carboplatin + Gemcitabine are the platinum-based chemotherapy regimen for first and second line, respectively.
Clinical parameters
Patients advance through treatment lines or exit the model according to the progression-free survival (PFS) and overall survival (OS) curves obtained from the corresponding pivotal clinical trials. The probability of progression to the next line of treatment was obtained from the difference between the PFS and OS curves, that is, in each cycle of the model patients who changed therapeutic line were the living patients (based on OS curve) who experienced progression (based on PFS curve).
The PFS curves of W&W and olaparib were developed according to the Kaplan-Meier (KM) curves reported in Study 19 for BRCA mutation positive patients11, where the median PFS was 11.2 months for olaparib and 4.3 months for W&W. This study included patients who had received at least two lines of chemotherapy, so the same curve was used in these alternatives for all treatment lines from the second onwards. For bevacizumab, the KM PFS curves reported in the GOG-21812 study were used for the first line and the OCEANS13 study for the second line.
Because the clinical trials of bevacizumab and olaparib showed no statistically significant differences in OS versus the comparator, a single OS curve was developed for each line, regardless of the maintenance treatment administered.
Therefore, it was decided to develop exponential curves based on the average of the median OS reported in the bevacizumab studies, since they were specific for first and second treatment lines, using the GOG-218 study (39.5 months) for the first line12 and the OCEANS study (33.3 months) for the second line13. In the absence of specific OS data for the third and fourth lines, and to simulate the decrease in survival in these lines, a correction factor was applied to the median OS calculated in the previous line. For this purpose, the same reduction in OS that was reported in the study by Hanker et al. was assumed14. Therefore, applying a correction factor of 64% with respect to the second line and 79% with respect to the third line, the calculated median OS in the third and fourth lines were 21.3 and 16.8 months, respectively. It was assumed that all patients that progressed beyond the fourth line would undergo palliative care for five month and until death.
Resource use and costs
The analysis only included direct costs (€ 2017): pharmacological, administration, adverse events (AE) associated with chemotherapy and the cost of BRCA gene testing (in the scenario with olaparib).
The ex-factory price15 was used to calculate the pharmacological costs, applying the corresponding deduction according to RDL 08/2010 (7.5% for bevacizumab and 4% for olaparib)16. The dosage recommended in the summary of product characteristics for each product (used in respective clinical trials as well) was used9, selecting the most economical packaging and considering the optimization of vials. To calculate intravenous (IV) doses, a mean weight of 73 kg17, as reported in the OCEANS study for patients with OC (close to that reported in Study 19-mean of 73.3 kg-), and a mean body surface area of 1.76m2 were used (calculated from the weight and height reported by the National Institute of Statistics for the Spanish population). The unit cost of one IV administration was € 263.4618. The estimated monthly (4 weeks) costs were between € 870.01 and € 1.402.36 for platinum-based chemotherapy, € 4,648.87 for bevacizumab and € 4,780.80 for olaparib as maintenance treatment (Table S4) (Supplementary Material).
Table S4 Monthly cost of doublet chemotherapy and maintenance treatments
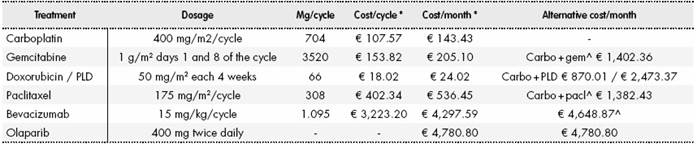
Carbo: carboplatin, Gem: gemcitabine, Pacl: paclitaxel, PLD: pegylated liposomal doxorubicin. The price used is the ex-factory price consulted in the Botplus, applying the deduction of RDL 8/2010 to May 2017 for bevacizumab (7.5%) and olaparib (4%). The mean weight used for the calculation was 73 kg and the mean body surface was 1.76 m2. In all cases, where possible, optimization of vials was considered. * One cycle consists of 21 days (3 weeks) and one month consists of 28 days (4 weeks).
^ Includes the cost of IV administration of € 263.46 per infusion.
The duration of treatment considered was 4.5 months (6 cycles) for doublet chemotherapy and until progression (according to PFS) for maintenance treatments (bevacizumab or olaparib). In the case of palliative treatment, a cost of € 838.71€ was considered for the 5 months prior to death19.
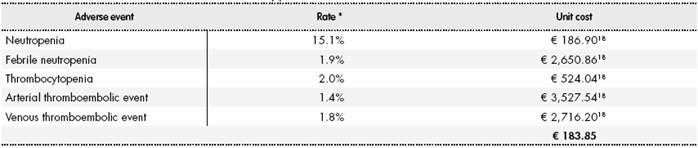
The cost of AE due to chemotherapy (with a frequency > 1% in the ICON7 study20) was calculated as the weighted mean of the unit cost of each event18 and the percentage of patients who experienced it during the study. The weighted cost attributed to AE due to chemotherapy was € 183.85 (Table S5) (Supplementary Material).
Sensitivity analysis
To evaluate the robustness of the results and determine their influence on the base case results, a univariate sensitivity analysis was performed, modifying individually the parameters with the greatest uncertainty. Therefore, parameters analysed were: the target population (± 5% of the parameters related to BRCA testing), correction factors for OS in the 3rd and 4th lines (± 50%), market shares of olaparib in the 2nd 3rd and 4th lines (± 20%), alternative parametrization of PFS curve for olaparib (log-normal distribution) based on Hettle et al. 22, duration of maintenance treatment (maximum duration of 16.5 months for olaparib and 15 months for bevacizumab in the 1st line9), weight and body surface area (± 20% and ± 10%, respectively), olaparib dose (mean 659.7 mg in Study 1911), chemotherapy cost (-25% and + 25% considering brand PDL) and the unit costs of BRCA testing and IV administration (± 50% and ± 20%, respectively).
Results
The results of the base case analysis show that the use of olaparib would generate an additional cost for the NHS of € 5 million in the third year, remaining stable until the fifth year. Although the economic impact of the cost of maintenance treatment and BRCA testing is positive, it was partially offset by the cost of chemotherapy and their related AEs, and the cost of palliative care, that were lower in patients with olaparib than in patients without olaparib (Table 1). In Supplementary Material (Table S6) shows cost breakdown by treatment line, considering only drug and administration costs.
Table 1 Overall results of the scenarios without and with olaparib and the resulting budgetary impact
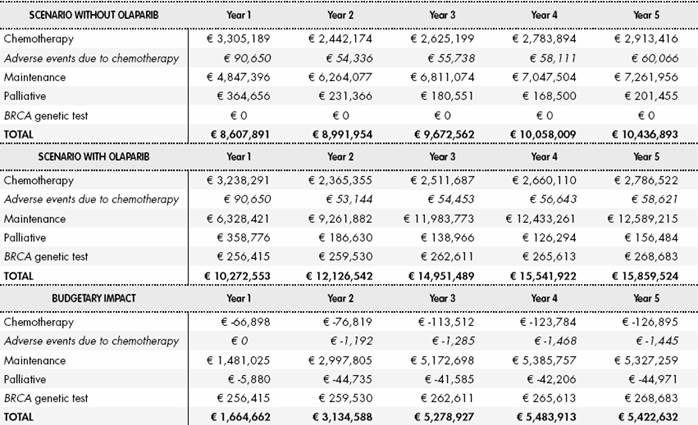
Table S6 Results for drug and administration costs, breakdown by treatment line (euro x 1,000)
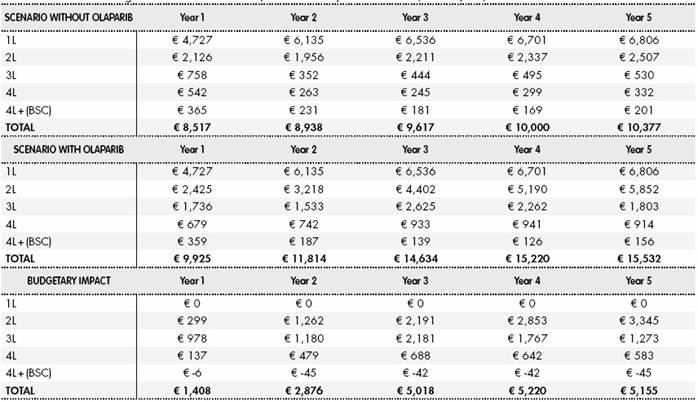
BSC: best supportive care
Cost of AE due to chemotherapy and testing cost for BRCA mutation are not included in this table
These results are conditioned by the clinical benefit provided by olaparib, since its use prolongs the PFS in comparison with the W&W alternative. In this context, after 5 years, it is estimated that 6% more women (167/584 vs 131/584) would remain free of progression in the second line of treatment in the scenario with olaparib, meaning that patients remain in early treatment lines for longer and the use of subsequent chemotherapy is delayed (Figure 2).

Figure 2 Percentage difference in the distribution of patients between treatment lines in the “with olaparib” scenario with respect to the “without olaparib” scenario. BSC: Best supportive care.
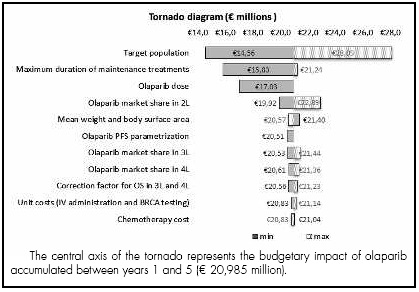
The results of the sensitivity analysis show that the greatest variability in the base case results occurred in the calculation of the target population, with changes of around 30-35% with respect to the base case. The remaining parameters analysed had a more moderate influence, ranging from 25% for the duration of maintenance treatment to 1% for the unit costs of BRCA testing and IV administration (Figure 3).
Discussion
Historically, ovarian cancer has been treated as a single entity, being surgery and the combination of platinum-based chemotherapy the gold standard. Even so, relapse rates remained high, resulting in multiple treatment lines, with disease-free intervals becoming shorter, and eventually resistance and reduced survival.
Today, in the era of personalized medicine, there is a demand for target treatments specifically for a population previously identified through a predictive biomarker of response, ensuring the patients who benefit most from treatment are selected.
The recent approval of olaparib for treatment of patients with BRCA-mutation positive HGSOC is, thanks to its PARP inhibition mechanism, an innovative new therapeutic option for BRCA-mutation positive OC, since it allows the selection of those patients who benefit the most and therefore it leads to a more efficient use of economic resources23. As maintenance therapy, olaparib can prolong the duration of tumour remission and thus increase the time to progression, as well as favouring the control of disease-related symptoms, and delaying the need for subsequent lines of chemotherapy, thus maintaining or improving the quality of life.
There are few economic evaluations in OC, although in the recent years the number of pharmacoeconomic studies published following the approval of olaparib24 and, in greater numbers, bevacizumab, has increased25-28. The majority of these studies are cost-effectiveness studies using Markov models to compare the costs and long-term effects of introducing olaparib or bevacizumab as maintenance treatment for OC. No budget impact studies have been identified in this context.
Therefore, the present analysis is the first to determine the economic impact of the introduction of olaparib as maintenance therapy in different OC treatment lines. In addition, by modelling the patient flow between successive treatment lines based on the PFS and OS, the analysis allows to estimate the proportion of women who might benefit from receiving effective maintenance therapies in the short term, delaying subsequent lines of chemotherapy.
In economic terms, the results of the analysis show that the scenario with olaparib would generate an additional cost for the Spanish NHS of € 1.6 to € 5.4 M from the first to the fifth year. This is because olaparib can be used in patients where the only option was W&W until progression, which did not involve pharmaceutical expenditure. In addition, considering that the total expenditure on cancer drugs in Spain in 2016 was € 1,920 million29, the additional cost of introducing olaparib would represent 0.09% of it, in the first year and 0.28% at five years.
Based on the study results, olaparib provides collateral savings despite the higher pharmaceutical costs because a greater percentage of patients stay in early therapeutic lines for longer, incurring less costs associated with the administration of subsequent chemotherapy and palliative treatments. At the same time, it is expected that the increased pharmacological costs associated with olaparib could have been mitigated if other types of direct costs were included in the analysis, such as medical visits, hospital stay and emergency admissions, as well as indirect costs due to lost work productivity of the patient or caregiver. In Spain, Antoñanzas et al.30 calculated the direct and indirect costs associated with various types of cancer. Although they did not include OC, they did report the results in cervical cancer, which amounted to € 49M per year in direct costs and € 43M per year in lost productivity. An Italian study by Angioli et al.31 estimated that the mean annual cost for each caregiver during the first-line treatment of OC was € 10,981, with 3% of total work days lost.
The present analysis has some limitations, including those inherent to pharmacoeconomic models analysing successive lines of treatment, which can make it difficult to correctly represent clinical reality. In this respect, due to the lack of efficacy data conditioned on previous treatments, reported data on the efficacy of maintenance therapies according to the treatment line (in bevacizumab) and assuming the same efficacy for all the lines on olaparib were used. The efficacy of some these drugs might vary according to previous treatment or the time elapsed since the previous treatment.
Another limitation in the modelling of the OS is that we used an exponential parametric approach to construct the curves, allowing adjustment according to the median values. In addition, since specific survival data for each line was not available, correction factors were applied to avoid overestimating the number of women in the most advanced lines.
Additionally, model compares indirectly patients treated with olaparib (based on characteristics of Study 19) and patients treated with bevacizumab (based on characteristics of OCEAN study), what supposes another limitation of the analysis, due of the absence of Matching-adjusted indirect comparisons or a network meta-analysis.
A further limitation inherent to budget impact analyses is the future market shares of the treatments. In the present analysis, although real market data were used to approximate the current utilization rate, establishing the distribution of the maintenance therapies used over five years results in uncertainty that has repercussions, in absolute terms, in the final results of the analysis.
Another limitation is the lack of a national cost database. Therefore, the unit cost of some parameters, such as the genetic test to identify BRCA mutations or intravenous administration, may vary between autonomous communities and even between centres. With regard to the types of costs included, as previously mentioned, no other direct costs associated with disease management were considered. In addition, to simplify the model, only the costs of AE related to chemotherapy were considered, since this was the only common treatment with a fixed duration, regardless of subsequent maintenance therapy. The rates of AE associated with maintenance treatments after chemotherapy are not high, and therefore the weight they would have on the model’s economic performance would have been minimal (< 1% of the total budgetary impact).
To evaluate the uncertainty associated with these limitations, a univariate sensitivity analysis was performed on the values considered in the base case and the assumptions made. This showed the parameters related to the calculation of the target population had the greatest impact on the result. Anyway, a further validation of clinical outcomes presented in this analysis would be needed.
In conclusion, improving OC treatment continues to be a challenge, mainly due to the late diagnosis of the disease and the poor efficacy of conventional treatments. The introduction of new targeted treatments is an important advance in the management of maintenance treatments in selected patients, albeit at the expense of an increase in health expenditure. In this context, our study shows, based on a non-adjusted indirect comparison, that olaparib used as maintenance therapy after a second and successive lines of chemotherapy, results in fewer women progressing and more remaining in the initial lines, thus delaying the need for successive chemotherapy lines that worsen their quality of life. The study results show that the use of olaparib as maintenance treatment in women with BRCA-mutation positive HGSOC would have a reasonable and moderate economic impact on the NHS, based on the total expenditure on cancer drugs in Spain.
Contribution to scientific literature
This study provides an estimate of the budgetary impact of olaparib in patients with BRCA mutation-positive high-grade serous ovarian, fallopian tube, or primary peritoneal cancer. Data is provided on its overall impact as well as by treatment line. It shows that women under maintenance treatment with olaparib would remain in initial lines, thus delaying the need for subsequent lines of chemotherapy.