Introduction
In Spain, there was a lack of awareness of the risks associated with the use of medical gases (MG) until their recent approval as medicines (Spanish Royal Decree 1800/2003, December 26)1. Many health organizations have developed strategies to increase the safe use of medicines, but the application of specific strategies to ensure the safe use of MGs has been very limited. Some studies have provided specific recommendations to promote correct storage and, in general, the safe use of MGs in hospitals2-7. The only article on each stage of the MG management procedure in hospitals was prepared by the Spanish Society of Hospital Pharmacy8. However, this publication did not include the prescription stage or address training and informing health professionals, nor did it establish how the entire process would be applied in practice.
Numerous studies have demonstrated that the major cause of adverse events in the provision of healthcare is the use of medication9-14. According to a report issued by the American Food and Drug Administration, between 1997 and 2001, errors related to the use of MGs in the United States resulted in 7 deaths and 15 serious injuries15. Therefore, it is crucial to consider MGs as medicines and to raise awareness among the professionals involved in their management of the consequences that may result from their incorrect use.
Based on the analysis of a sentinel event, the objective of this study was to redefine the entire process of MG use in hospitals, including aspects related to logistics and training.
Methods
This study was conducted in a 1,300-bed university tertiary hospital employing more than 8,000 staff providing healthcare to approximately 350,000 people.
A paediatric hospitalization unit inpatient experienced a sentinel event that involved the incorrect administration of an MG. As a result, the patient went into respiratory arrest and was admitted to the Intensive Care Unit (ICU). Neurological monitoring was performed to confirm the absence of injury. After the sentinel event was detected, the Risk Management Functional Unit (RMFU) of the hospital was notified. The RMFU is made up of experts on safety from various services, including 2 other pharmacists from the hospital. The president of the RMFU is also the head of the Pharmacy Service. The general objective of the RMFU is to increase the quality of healthcare and patient safety, and thus the decision was taken to conduct a root cause analysis (RCA) of the event.
Root cause analysis
1. A multidisciplinary team was formed comprising the head of the hospital’s Mother-Child Unit, an assistant physician from the Paediatric Intensive Care Unit, an assistant physician from the Paediatric Hospitalization Unit, an assistant physician from Preventive Medicine and Quality Management, who was also member of the RMFU, and the pharmacist from the Mother-Child Unit.
2.The team followed the classic methodology of RCA16 and established the sequence of events based on which it identified possible failures and/ or deviations from established procedures and their causes. A sequential process of structured questions was used to identify the possible causes of failure and determine the latent errors underlying the sentinel event.
The team proposed areas for improvement to address these errors and thus define a program that would guarantee the safety and quality of MG use. These improvements were prioritized taking into account the magnitude and consequences of the failures and the feasibility of the proposed measures. The improvement measures were developed to prevent the repetition of similar events. A specific member of staff was assigned to implement these measures along with their deadlines.
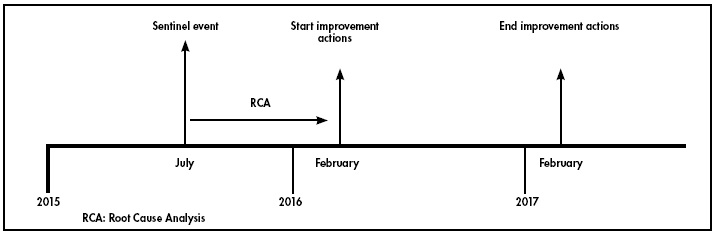
The RCA was conducted between July 2015 and February 2016 (see Figure 1).
Results
The RCA identified 9 errors that were made along the timeline that led to the sentinel event (Figure 2).
Organizational improvements
Improvement actions were defined for each stage of the use process, and a specific member of staff was assigned to each of them and implement them within a given period (Table 1). All actions were simultaneously implemented in all the clinical units of the hospital.
Storage. The study hospital houses MGs in 4 storerooms which contain gases for clinical and industrial use. Firstly, the 4 storerooms were reorganised by using physical barriers to separate the gases for clinical use (oxygen, heliox, nitrous oxide, nitric oxide, and medicinal air) from those for industrial use (argon, carbon dioxide, helium, sulphur hexafluoride, and nitrogen) used for the maintenance and operation of hospital machines. The different gases were placed in independent physical spaces.
In line with current legislation (RD 1800/2003)17, identification signs were put up with the name of the gas and a description of the bottle to assist in its correct identification by the staff responsible for its transfer to the clinical units.
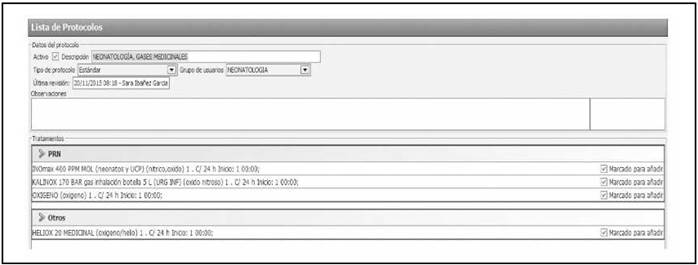
Prescription. MGs were included as medicines so that they could be prescribed through the hospital’s Computerized Physician Order Entry system. Six protocols were created for their prescription in this software: neonatology medical gases (oxygen, heliox, nitrous oxide, and nitric oxide), paediatric medical gases (oxygen, heliox, and nitrous oxide), paediatric ICU medical gases (oxygen, heliox, nitric oxide, and nitrous oxide), and gases used in emergency paediatric bronchospasm (oxygen), emergency paediatric bronchiolitis (oxygen), and emergency paediatric laryngitis (heliox). Figure 3 shows one of these protocols included in the assisted electronic prescribing program. These protocols included recommendations on the form of administration and warnings related with patient monitoring. Thus, manual prescription and its associated risk of error were avoided, and variability among hospital staff was reduced when prescribing these gases. All the hospital staff were informed of the action taken.
Validation. As with all other prescribed medications, the inclusion of these MGs in the Computerized Physician Order Entry system allows the pharmacists to review their prescription and correct use such that the specific requirements for each MG are met. Thus, the correct indication, correct flow, and duration of treatment are confirmed.
Dispensing. To ensure safety during the dispensing stage, a transfer protocol was developed and implemented that included the identification of all staff with access to the gas store, their continuous training, and the use of an order delivery note that has to be signed by the person making the delivery and the person receiving the order. Figure 4 shows the MG transfer process.
Administration. The MG supplier was asked for information on the manometers used to administer each gas. Although each clinical gas has a different type of connection system, it may be the same as that used for industrial gases. Thus, it must first be verified that the fitting is correct for the gas used before it is administered.
Training. Firstly, in order to promote the safe use of MGs, 72 posters with recommendations for their correct identification and administration were put up in the clinical units. Figure 5 shows the poster used.
The poster shows how the bottles for clinical use should be identified according to current legislation (RD 1800/2003)17:
- A label that shows the name of the gas, batch/sub-batch, and expiration date.
- A “banana” label with the risk and safety characteristics of each product, recommendations for use, hazard pictograms, and the composition of the packaged gas.
- The colour codes: oxygen (white shoulder and body), medicinal air (white shoulder/body and black stripe), nitric oxide (aquamarine shoulder and white body), nitrous oxide (dark blue shoulder and white body), and heliox (white body/shoulder and brown stripe).
- A Red Cross. This symbol shows that the gas is for clinical use.
- The letter “N” marked twice on diametrically opposite points on the shoulder. The colour of the letter differs from the colour of the shoulder. The “N” confirms that the bottle complies with the latest legislation.
In addition, our hospital runs two 20 - hour patient safety courses which are routinely given to all the hospital staff. This course includes a specific unit on the correct use of medicines, including MG, as well as medical devices. The course emphasises the need to fulfil the 7 “rights” (right patient, right time, right medication, right dose, right administration route, right documentation, and right information).
Moreover, new personnel receive a welcome pack that includes all the documentation related to the safe use of MGs, thus ensuring that the staff of the hospitalization units know which gases are used in the unit and the protocol to be followed for their use.
Discussion
Following the RCA of a sentinel event, we redefined the use process to guarantee the safe use of MGs in our hospital. A set of improvement measures have been implemented that address all stages of the medication use process: storage, prescription, validation, dispensing, administration, and training. One of the hospital pharmacists, who was also member of the RMFU, was appointed to ensure adherence with all the implemented improvement measures and to provide the RMFU with a semiannual report on their monitoring. In the future, any related incidents will be monitored to verify the effectiveness of the implemented measures and their possible adjustment.
Studies on errors in MG use have mainly addressed improvements in storage as well as in nurse training because nurses are the health staff responsible for the administration of MGs8,12. However, we used the RCA methodology to analyse all stages of the medicine use process and integrated them within this process, including the prescription, validation, and dispensing stages. Thus, all the staff who use MGs are now involved in this process, thereby promoting and ensuring a culture of safety in the use of MGs in our hospital.
Several international studies have reviewed current legislation on the handling and labelling of industrial and medical gases, and have emphasised the differences between them9-12,14. In line with these studies, we have circulated the regulations (RD 1800/2003)17 applicable in our setting to ensure adherence and avoid errors due to their incorrect identification.
Herve-Bazin et al.6 analysed medication errors in the use of MGs reported by health professionals in France. Most of these errors were due to confusing oxygen with oxygen/nitric oxide mixture, causing severe adverse effects and even death in some patients. We were unable to analyse the errors reported in our hospital because, as mentioned, until the measures described were implemented, there was a lack of awareness of hazards in the use of MGs that led to these errors being underreported. The French study suggested the creation of a poster that included general recommendations on MGs with the aim of informing healthcare professionals of the hazards posed by their use13. Our poster is similar to the one suggested in that study. However, their study did not establish specific measures that would guarantee safety throughout the entire process, whereas such measures were implemented in our hospital.
The MG use process defined in the present study is applicable to any health institution without incurring significant cost. However, the situation of each stage of the MG process should be determined and the staff in charge of each stage should be identified in order to implement the improvement measures applicable in each case.
One of the main limitations to the application of our approach in other hospitals is the need for Computerized Physician Order Entry system that can incorporate protocols for the correct use of MG. Although not all Spanish hospitals have such software, a survey conducted in 2015 by the Spanish Hospital Pharmacy Society 2020 working group showed that 94% of hospitals already had this type of software.
On the other hand, there is a need for personnel to train others in the use of such software. However, online eLearning training systems are currently available that can be accessed by any professional.
Once the use process has been consolidated over the next 2 years, a failure mode and effects analysis will be conducted to assess the risks involved in each of the stages of the implemented use process, thus improving the implemented measures.
In conclusion, MGs should be considered as medicines as such and thus the risks associated with their use should be taken into account. To this end, an adequate MG use process should be implemented in the hospital setting that guarantees the safety of patients, and an appropriate training program should be set up for the professionals involved in their use.











 texto en
texto en