Introduction
Parenteral nutrition (PN) is essential to the survival of neonates whose nutritional requirements cannot be met via the oral route.
Scientific societies, such as the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the European Society of Clinical Nutrition and Metabolism (ESPEN), provide specific guidelines for the prescription of PN in this group of patients1,2. However, PN is associated with a high risk of adverse effects, such as infections or metabolic imbalances, which can put intrinsically very vulnerable patients at risk3. Any prescription error, compounding error, or microbiological contamination could probé lethal for such vulnerable patients.
Furthermore, the complexity of compounding PNs constitutes a risk in itself. The main causes of this complexity are the handling of a large number of compounds in very small volumes, physicochemical stability issues, and a high risk of contamination during the compounding process4.
In recent years, the introduction of semi-automated systems for the compounding of PN mixtures has strongly improved traceability and the safety of the process5 6-7. However, the implementation of these systems for compounding PN for low- birth-weight preterm neonates is challenging because it entails dealing with components of less than 1 mL in volume. For this reason, PN for preterm neonates is typically compounded by hand. Thus, it is essential to implement quality controls to reduce the likelihood of errors and to guarantee patient safety. In-process controls, such as double-checks or systems that ensure the traceability of the ingredients, are valid alternatives in the absence of semi-automated compounding systems8. Microbiological testing is also needed to ensure that the final product is sterile. A gravimetric test with an acceptance margin of 5% for the final weight of the bags is the most widespread type of test of the finished product8 9-10. However, given that most of the compounds are used in very low volumes, its ability to detect compounding errors is quite limited.
Parenteral nutrition typically contains amino acids, glucose, lipids, electrolytes (sodium, potassium, calcium, phosphorus, and magnesium), trace elements, vitamins, and drugs such as carnitine and heparin. The biochemical analysis of the composition of PNs could be an effective control method to ensure the correct composition of critical compounds, such as glucose or some potentially dangerous electrolytes, and to prevent compounding errors. The concentrations of these compounds in PN can be measured by adapting conventional techniques used in clinical laboratories to measure their concentrations in biological samples. This is a simple alternative that is available in almost all hospitals. Emergency laboratories already work within short response times, and the measurement procedures used can rapidly obtain results at a very low cost.
The aim of this study was to describe the validation of the procedure used in the biochemical testing of PN for preterm neonates, and to assess the impact on patient safety of its implementation in a tertiary hospital.
Methods
Validation of the procedure
The most clinically relevant compounds measured in this process were glucose, sodium, potassium, magnesium, and calcium.
They were measured in the emergency laboratory of our hospital using the Dimension EXL automatic analyser (Siemens Diagnostics), which allows us to measure, for example, glucose and electrolyte concentrations in plasma and urine.
Glucose and calcium were measured by molecular absorption spectrometry using the Dimension EXL (GLUC Ref DF40 and CA Ref DF23A, respectively). Sodium and potassium concentrations were measured by the indirect potentiometry method using the Dimension QuikLYTE (Ref S600). The procedures used to measure calcium, magnesium, sodium, and potassium concentrations in PN were not modified, whereas glucose was measured after diluting the PN samples with distilled water at a ratio of 1:20.
To validate the measurement procedures, we used 35 samples of lipid-free PN, which were replicas of real samples and were compounded using double-checks to guarantee their correct composition. The in-process double-checks and the use of volumetric and weighing material with negligible measurement uncertainty allowed us to assume that the samples used for validation were free of compounding errors. Measurement inaccuracy was calculated using Passing-Bablok non-parametric linear regression to analyse the difference between theoretical concentrations and measured concentrations11,12.
In order to interpret the results obtained in daily practice, we also reached a consensus with neonatologists on the clinically acceptable margins of error for each compound.
Assessment of the implementation
Risk analysis
The failure mode, effect, and criticality analysis (FMECA) method was used to quantify the pre-implementation and post-implementation risks of the process.
This analysis was based on a global redesign of the paediatric PN prescription and compounding process, which included an electronic prescription program and a double-check system in addition to the implementation of biochemical mixture tests.
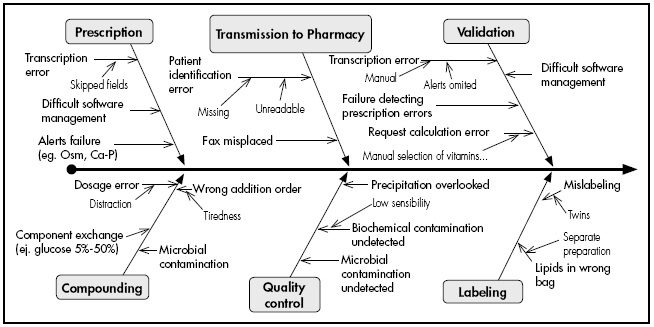
A multidisciplinary team of pharmacists and pharmacy technicians with expertise in compounding paediatric PN used an Ishikawa diagram to identify all possible errors during the prescription, validation, and compounding of the mixtures. Subsequently, they assigned a value to each possible error in terms of its likelihood (from 1 to 10), severity of its consequences for the patient (from 1 to 10), and difficulty in detecting the error during the process (from 10 to 1). These three items were multiplied to obtain the criticality index (CI), which quantifies the magnitude of the risk of the process.
The difference between the pre- and post-implementation CI provides an estimation of the impact of the implementation on overall risk reduction.
Assessment of effectiveness
We assessed all paediatric PNs compounded in the first 2 years post-implementation. The mixtures did not contain lipids because they are administered separately in our hospital. We recorded the following variables: patient weight on day PN started, gestational age, and duration of nutrition. We also quantified the response time from the moment a laboratory analysis was requested until the time of its validation in the hospital’s computer system, as well as the mean cost of analysis. Finally, we assessed all preparations with out-of-range results and analysed their causes and possible impact on patient safety.
Results
Validation of the procedure
The neonatologists used a theoretical concentration of ± 10% as the clinically acceptable range for the components under analysis, although larger deviations could be acceptable depending on the characteristics and clinical situation of the preterm neonate.
Table 1andTable 2show the mean concentrations of the samples used for validation and the regression lines for each compound, respectively.
Table 2. Regression lines for the relative inaccuracy of the different measurement procedures and their correlation coefficients

a: proportional error; b: constant error; CI: confidence interval; Conc Me: measured concentration; Conc Theo: theoretical concentration.
The regression analysis obtained a correlation coefficient of more than 0.975 for all parameters. No systematic measurement errors were found for glucose and calcium. A positive systematic proportional error of 3.7% was found in the case of potassium; however, this result was not considered to be clinically relevant. A positive systematic constant error of 3 mmol/L was found in the case of sodium. This level of error is irrelevant at high concentrations, but can be significant at small concentrations. Thus, sodium was not included in the biochemical test. A mixed systematic error was found in the case of magnesium, with a proportional error of 11.3%. This error is higher than the acceptance range (± 10%), and so magnesium was also not included in the biochemical test.
The relative inaccuracies observed for magnesium, sodium, and potassium could be due to the effect of the different matrix used for the samples (plasma). Therefore it was considered that they could be corrected with the use of suitable calibrators. However, this corrective measure was postponed because the aim of implementing this control method was to make available a technique that was easy to integrate into the routine of the hospital’s emergency laboratory without the need for excessive manipulation of the samples under analysis. In this way, the procedure closely resembles that used for biological samples. Furthermore, excellent correlation coefficients were found for glucose, calcium, and potassium, which are considered to be the most critical compounds.
The biochemical test was implemented within the standard routine of the pharmacy service and laboratory service by the addition of automatic calculation software to the hospital’s computer system (SAP®). The request form indicated the volume of each of the ingredients to be determined and the total volume of the bag. Based on these data and the starting solution concentrations, the theoretical concentration of the ingredients was calculated automatically. The measurements obtained by the analyser were automatically uploaded to the hospital’s computer system, such that the difference in percentage between expected and measured concentrations could be visualised (Figure 1).

Figure 1. Example of results as shown in the hospital software. Gluc, glucose; Na, sodium; Ca, calcium; K, potassium.
Assessment of the implementation
Risk analysis
As shown in the Ishikawa diagram inFigure 2, a total of 31 possible errors were identified throughout the process. A CI was calculated for each of the 31 possible errors based on their likelihood, severity, and difficulty of detection. The pre- and post-implementation global CIs were 4.964 and 1.715, respectively. This reduction represents a 65.5% decrease in overall risk, of which 11% was due to the implementation.
Assessment of effectiveness
A total of 1734 PN bags were used in the analysis, involving 218 neonates with a gestational age of 30 ± 4 weeks. On the first day of PN, mean weight was 1.35 ± 0.8 kg. In total, 94 patients (43%) weighed less than 1 kg. The mean duration of PN was 7.95 days.
The mixtures used had a mean of 12.4 ± 1.1 compounds, of which 50% had a volume of less than 1 mL. The PN bags analysed had a mean total volume of 162.2 ± 27.1 mL. This volume includes the 50 mL of purge volume that is added to each bag. All compounded PNs were analysed and 5-mL samples were sent to the emergency laboratory.
The mean response time from reception of the sample at the laboratory to validation of the result by the software was 55.3 ± 7.4 minutes. The estimated cost of each determination was € 0.25.
Of the 1734 samples analysed, 58 (3.3%) were found to be out-of-range for glucose, calcium, and potassium (± 10%).
In 34 of the 58 cases, the deviation was slightly above the acceptance range (between 10% and 15%); however, the neonatologists considered that the deviation would not have any clinical repercussions, and so the compounding was accepted as correct. In 17 cases, the out-of-range result was identified either as a transcription error when using the software to enter the request, or as an identification error regarding the patient for whom the request was made. Finally, the 7 remaining cases were identified as potentially dangerous compounding errors, and so the bags were discarded and the PN was compounded again. Of these 7 errors, 3 were related to the calcium concentration in the sample, 3 to the potassium concentration, and 1 to the glucose concentration.
Discussion
The complexity of the mixtures and the fragility of the patient make the prescription and compounding of paediatric PNs for preterm neonates a critical process that requires continuous review to guarantee safety.
The biochemical analysis of nutrition can provide great added value to this process by ensuring the correct composition of critical ingredients, such as glucose or potassium. The fact that it is not a standard practice in hospitals may be due to either the difficulty of integrating it into daily routines or its high cost, depending on the measurement instruments used.
However, tests are randomly conducted on a specific percentage of PNs. This study shows that it is feasible to implement biochemical test for all PN bags at a genuinely affordable cost, due to interdisciplinary collaboration between the emergency laboratory and pharmacy services. The response time, which is less than 1 hour in most cases, means that possible errors can be detected before the time of administration and the PN can be compounded again if needed.
Although the risk analysis showed that the implementation of a biochemical test reduces the overall risk inherent to the process, other measures, such as electronic prescription or the introduction of in-process controls (e.g. double-checks in the present study), could have the same or an even greater effect on risk reduction.
It is relevant to note that despite the introduction of the new measures, a certain level of risk always remained (34% in our case). These risks should be studied, their causes clearly determined, and suitable measures implemented with the aim of reducing or eliminating them.
Although the results of the FMECA cannot be extrapolated to other hospitals, various institutions such as the Joint Commission or the Council of Europe13,14have recommended the integration of risk analysis into standard practice in hospitals as a support tool for continuous improvement.
Although we did not collect data on PN medication errors that reached the patient before or after the implementation of the biochemical test, we consider that the results corroborate the benefits of the intervention in relation to risk reduction in the PN compounding process and error avoidance.
The data show that the study population mainly consisted of preterm neonates weighing less than 1.5 kg, whose indication for PN is, by definition, their prematurity. The challenge of compounding these PNs resides in the high number of ingredients and small volumes involved in their preparation. This wellknown problem has been approached in various ways. These approaches include the appropriate training and periodic assessment of the professionals who compound PNs, although other authors have investigated the advantages of automation or the use of standardized mixtures15 16-17. Future research will undoubtedly determine the performance of these options in clinical practice. Until then, interdisciplinary teams responsible for neonatal PN should continue to innovate and apply continuous improvement strategies to meet the challenge of improving the safety of the compounding process.
In conclusion, the biochemical test of glucose and electrolytes is an efficient and reproducible method. This kind of biochemical testing can be used to analyse critical ingredients in all PNs. The results would be known before the administration of PN, thus preventing potential compounding errors from reaching the patient. In our 2-year experience, the use of biochemical testing allowed us to detect 7 compounding errors with potentially severe consequences in these neonatal patients.











 texto en
texto en