Introduction
The treatment of rheumatoid arthritis (RA) and spondyloarthritis has improved dramatically with management by biologic agents (tumor necrosis factor inhibitors (TNFis), such as infliximab (Ifx), adalimumab (Ada), etanercept (Eta), golimumab (Goli), and certolizumab pegol (Certo), and other drugs such as tocilizumab (Toci), rituximab (Ritu), and abatacept (Abat). Some of these drugs have been approved for RA, and/or spondyloarthritis by the European Medicines Agency and the US Food and Drug Administration1. Spondyloarthritis is a group of several related but phenotypically distinct disorders that includes arthritis related to inflammatory bowel disease, psoriatic arthritis, ankylosing spondylitis (the prototypic and best studied subtype), and others, but the pathologies with major clinical phenotypes are axial spondyloarthritis (SpA) (radiographic SpA called ankylosing spondylitis, and nonradiographic SpA)2 and psoriatic arthritis (PsA)1. Despite relevant evidence supporting short - term TNFi efficacy and safety from randomized controlled trials3-5, data on their long-term effects are limited.
Persistence with treatment is important for effective disease management, especially in chronic diseases that can become more severe over time, such as autoimmune and inflammatory conditions. Treatment persistence is defined as the time period from initiation to discontinuation of therapy, and is measured by continuation rate, retention rate, and drug survival. Medication persistence refers to the act of continuing the treatment for the prescribed duration6. Drug retention has been found to be a reliable indicator of overall treatment effectiveness in observational studies, given that it is mainly determined by both drug efficacy and safety profiles7-12.
We performed a retrospective study to calculate the persistence of biological agents in daily clinical practice in our hospital. The aims of this study were to determine, over a period of eight years, the retention rate of first -line and second-line, of treatment with biological agents in patients with RA, SpA and PsA. The secondary objectives were to compare the retention rates of the various drugs for each pathology and to analyze the causes and rates of discontinuation of these treatments.
Methods
A retrospective observational study was performed in a hospital in Madrid. The study was approved by the Clinical Research Ethics Committee of La Paz University Hospital. The study included patients affected by RA, SpA, and PsA who started treatment with biological agents between January 2009 and December 2012. Biological therapies were dispensed at the Pharmacy Department in the hospital. Patients were followed until December 2016 and we determined the eight-year retention rate of the first- line and second - line biological drugs. However, due to the small number of patients taking second-line drugs for PsA, the retention rate for these drugs was not calculated. Drug survival was calculated in months (1 month = 30 days). Drug survival sub - analyses were conducted by stratifying the study population according to the diagnosis (RA, SpA, and PsA). We analyzed the type of prescribed biological drugs for three pathologies and the retention rate for each drug as well as for all drugs together.
Data were recorded in our software program (FarmaTools 2.5 Dominion) for drugs prescribed and dispensed to outpatients. Demographic and clinical data were obtained from the La Paz Database for Biological Therapies for Rheumatology, which was created by the Rheumatology Unit of the hospital.
In addition, we calculated the number and rates of patients who discontinued their first treatment, and the reasons for treatment discontinuation were analyzed.
Discontinuations were considered to have occurred when no consecutive reintroduction of treatment was reported or on the date when treatment was switched to another biological agent. Observations were recorded at the last registered visit to the pharmacy service, where biological agents were dispensed for the final time. The reasons for discontinuation were assigned to five categories: primary inefficacy (lack of response), secondary inefficacy (loss of response or failure), adverse events, remission, and other (including loss to follow-up, final treatment for other pathologies, and final treatment for other causes).
Statistical analysis
The clinical results are expressed as mean and standard deviation. All test were performed using IBM SPSS version 19. Differences in patients´ characteristics were examined using the chi-squared test for categorical variables and t- test for continuous variables. For statistical significance, p- values < 0.05 were considered significant. Persistence was expressed in months, and it was exported to an Excel table for statistical analysis using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). The retention rate was estimated using the Kaplan-Meier method. The median time (in months) of retention and the cumulative (patients-year) treatment exposure were calculated. These analyses were done on all biological drugs together and on each drug individually. The resulting curves were compared with the log-rank test.
Results
Our study included 132 patients with RA, 87 patients with SpA (58.6% with ankylosing spondylitis), and 33 patients with PsA who started their first biological agent between 2009 and 2012. The percentages of patients at the end of the study in December 2016 were 43.1%, 64.3%, and 72.7% in the RA, SpA, and PsA groups, respectively. Patients who discontinued the treatment were 75, 31, and 9 patients in RA, SpA, and PsA groups, respectively. There were no losses due to death or transfer to another hospital. The demographic and clinical characteristics as DAS28 (Disease activity Score for Rheumatoid Arthritis), SDAI (Simple Disease Activity Index), BASDAI (Bath Ankylonsing Spondylitis Activity Index), ASDAS (Ankylosing Spondylitis Disease Activity Score), CRP (C-reactive protein) and ESR (Erythrocite Sedimentation Rate) of the patients at beginning and at the end of the study are shown in Table 1. At baseline, the patients’ disease activity parameters were considered to be active, and at the end of study, these parameters were lower (p < 0.05).
Table 1. Demographic and clinical parameters of the patient at baseline and at the end of study

Data are expresed as mean (SD) for continuous variables and frequencies (percentage) for categorical variables. ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylonsing Spondylitis Activity Index; CPR: C-reactive protein; DAS 28: Disease activity Score for Rheumatoid Arthritis; ESR: Erythrocite Sedimentation Rate; HLA B-27: Human leukocyte antigen B27; SDAI: Simple Disease Activity Index. Statistical signification P < 0.05; *T test; **Chi-square test.
Inactive disease: ASDAS < 1.3, DAS28 < 2.6, SDAI ≤ 3.3, and BASDAI ≤ 2.
Moderate diasease: ASDAS >1.3 and < 2.1, DAS28 ≥ 3.2 and ≤ 5.1.
Low activity: DAS28 ≥ 2.6 and < 3.2, SDAI > 3.3 and < 11
Clinical control: BASDAI < 4 and Δ ASDAS ≥ 1.1
(Reumatol Clin. 2015;11:279-94; Reumatol Clin. 2011;7:113-23)
Patients received Ada, Eta, Ifx, and Goli as biological drug therapy in the SpA and PsA groups, and moreover Certo, Ritu, Abat, and Toci for those in the RA group. Patients started with Ada, Ifx, and Goli more frequently in SpA (35.6%, 34.4%, and 16.1%, respectively), with Eta, Ada, and Goli in PsA (36.3%, 27.3%, and 21.5%, respectively) and with Eta, Ada, and Toci in RA group (31.8%, 12.1%, and 11.3%, respectively).
All-drug survival
In this study, the median retention duration of biological agents for the first-line drugs, until the first switch (in months), was longer for PsA (> 70 months), and SpA (63.06 months, 95% CI 42.2-83.8) than for RA (30.9 months, 95% CI 13.1-48.3). Survival functions in months for RA, SpA, and PsA in first- and second-line drugs (p=0.002) are shown in Figure 1.

Figure 1. Survival functions in months for rheumathoid arthritis, spondyloarthritis and psoriatic arthritis. PsA: psoriatic arthritis; RA: rheumathoid arthritis; SpA: spondyloarthritis.
Survival rates for first-line drugs at the fifth and eighth years were 35.8%, and 22.7% for RA, respectively; 51.0%, and 37.3% for SpA, respectively; and 61.9%, and 56.3% for PsA, respectively (Figure 2).
Individual drug survival
Rheumatoid arthritis:
First-line biological agents:
Significant differences emerged in the survival distribution for each drug (p = 0.003). The median retention duration of Toci was 58.3 months (95% CI 32.4-84.2), followed by Eta at 44.0 months (95% CI 11.8-76.1, p = 0.79). Eta had more retention than other TNFi drugs:
34.9 months for Certo (p = 0.98), 16.6 for Ada (p = 0.021), and 7.7 for Ifx (p = 0.002). A pairwise comparison analysis of Toci versus other biological drugs revealed a statistically significant median retention with respect to Ada and Ritu (p = 0.015, and p = 0.009, respectively).
Second-line biological agents:
No differences were found for second-line treatment in survival drugs (p = 0.096).
Toci was the most persistent biological drug, with a median of 22.1 months (95% CI 10.1-33.9), followed by Abat and Ada with 11.0 (95% CI 0.0-25.4), and 8.0 (95% CI 5.8-10.4), respectively.
Spondyloarthropathies:
First-line biological agents:
In SpA, the three-year drug survival rate in our study was (64.4%) and the eight-year drug survival rate was (37.3%). No significant differences emerged in the survival distribution for all of the drugs for SpA (p = 0.586). Goli and Eta had greater retention than the other TNFi drugs, but they did not reach the median. The median retention duration for Ada was 63.0 months (95% CI 34.3-91.7), and for Ifx it was 50.1 months (95% CI 23.8-76.4).
Second-line biological agents:
Goli, as it happened in the first-line, was the most persistent biological drug. It did not reach the median reaching a drug survival rate of 71.1% at the third year.
The median retention of Ifx was 19.7 months, followed by Ada and Eta with 10.1 (95% CI 0.39-19.8) and 5.7 (95% CI 0.0-17.7), respectively. No significant differences were found (p=0.129). Our study showed a survival rate of Ifx (50%) and Ada (33.3%) at the second year, and Eta (16.7%) at the first year.
Psoriatic arthritis:
First-line biological agents:
The retention duration of the first, second (median), and third percentile until the first switch to other drugs, all together, was 78.1, 59.4, and 42.3 months, respectively.
Goli, Ifx, and Eta did not reach the median, and they had greater retention than Ada (median 59.4 months (95% CI 11.2-77.6)).
The three-year survival rate for all-drugs was 69.7%, and the eight-year survival rate was 56.3%.
The cumulative proportional drug survival at 7 years was 85.7%, 60.0%, 52.5%, and 44.4% for Goli, Ifx, Eta. and Ada, respectively.
Reasons for discontinuation
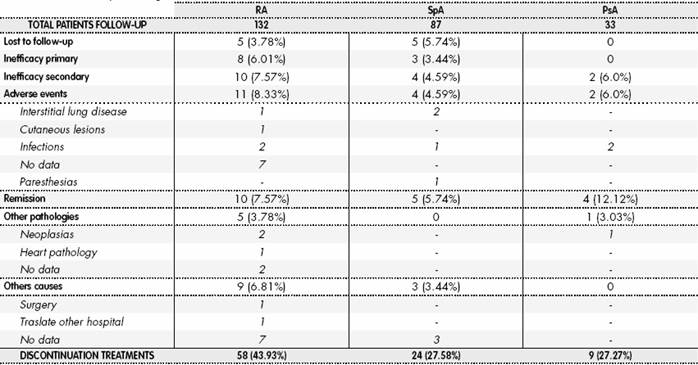
A total of 58/132 (43.9%), 24/87 (27.58%), and 9/33 (27.3%) patients stopped the first-line biological therapy in RA, SpA, and PsA, respectively. There were no losses due to death or transfer to another hospital. Discontinuation was due to primary inefficacy in 13.79% and 12.5% of patients with RA and SpA, respectively. Discontinuation was due to secondary inefficacy in 17.2%, 16.6%, and 8.3% for patients with RA, SpA, and PsA, respectively, and due to adverse events in 18.96%, 16.6%, and 8.3% in patients with RA, SpA, and PsA, respectively. Other causes and percentages of discontinuations are reported in Table 2.
Discussion
In this study, the median retention duration of biological agents for first-line treatment, until the first switch (in months), was longer for PsA (> 70) and SpA (63.06) than for RA (30.9), respectively. The survival rate in the fifth and eighth years were 35.8% and 22.7% for RA, respectively; 47.7% and 37.3%, for SpA, respectively; and 61.9% and 56.3% PsA, respectively. There were greater differences between the results obtained for the two first pathologies with respect to RA.
Our results on drug survival of TNFi treatments in SpA compared with RA were similar to those published by other authors13. In our study, we found differences in the discontinuation rate of biological agents when comparing first- versus second-line therapy with biological agents. The duration of TNFi treatment as second-line therapy was shorter than in the first -line (30.9 versus 14.0 months in RA and 63.07 versus 25.6 months in SpA). In line with these results, other studies13-15 also suggest that a second TNFi (involving only TNFis, such as Ada, Eta, and Ifx, but no other biological drugs) are effective, but generally for less time.
In our study, the duration of efficacy of TNFis in RA was 30.9 months in first-line. Similarly, Frazier et al. publish that in RA the median retention duration of TNFi are 36 months15. We observed a longer median retention duration for Toci and Eta (58.3 and 44.0 months, respectively) compared with other biological agents.
Recent studies report a lower discontinuation rate for Eta, mainly because it was better tolerated than Ada and Ifx16. We observed a similar retention duration for Eta when comparing our results with the Frazier et al.
report (44 months versus 45 months). However, drug survival times for Ada and Ifx in our study were much shorter (16.6 and 7.7 months versus 31 and 23 months, respectively).
It should be noted that all studies only compare these three TNFi drugs. To our knowledge, no other comparisons of all biologicals available for rheumatic diseases such as those in our study have been published15-19.
For second-line drugs, our study showed that Toci (median retention 22.1 months) was the most persistent, followed by Abat, Ada, and other TNFi treatments. Some observational studies compare the efficacy and retention of rituximab, Abat, and Certo as a second TNFi; however, they do not exceed one year of follow-up20-22. A systematic review conclude that switching to Abat, Ritu, and Toci are more effective than cycling TNFi in patients with an inadequate response to TNFis23.
In SpA, the three- year survival rate in our study (64.4%) was consistent with data reported in the Italian cohort MonitorNet (69%)24, and was lower than data reported by the Spanish Society of Rheumatology (76%)25 or reported by Favalli et al. (72%)7.
The eight-year survival rate for SpA in our study (37.3%) was lower than that of Favalli’s study (57.2%). This difference is probably due to the smaller number of patients in our study (88 patients) compared with theirs (316 patients).
If we analyze the order in the first-line drug survival after 5 years, Goli and Eta (64.3% and 58.3%, respectively) were more persistent than Ada and Ifx (51.6% and 42.7%, respectively). The persistence concurs with data reported by Favalli et al., who find an estimated proportion of patients maintaining Eta, Ada, and Ifx of 79.9%, 76.2%, and 64%%, respectively, after 5 years. However Goli is not included in their study period, as only 3 TNFis were available in Italy7. The survival order in Favalli’s study changed after eight years (76.2%, 69.2%, and 50.7% for Ada, Eta, and Ifx, respectively). Our results were different for Goli, Eta, Ifx, and Ada (64.3%, 58.3%, 34.1%, and 29.5%, respectively). Favalli et al. explain their results for Ada persistence by considering the frequent coexistence in SpA of extra-articular manifestations such as uveitis and inflammatory bowel disease, in which TNFis have been proven to be more effective than Eta, thus affecting drug survival7.
For second-line drugs for SpA, our study showed that Goli at the third year was again the most persistent (71.1%), followed by Ifx (50%), and Ada (33.3%) at the second year, and Eta (16.7%) at the first year. To our knowledge, no other studies have been published in which the rate survival in the second -line drugs for SpA has been calculated, such as those of our study. Favalli et al. have been published a study in which second-line Goli shows an overall better 2-year drug survival compared with Ada and Eta26.
For first-line drugs for PsA, the three-year survival rate in our study (69.7%) was lower than that reported by the Spanish Society of Rheumatology (73%)25 and higher than that reported by the Italian cohort MonitorNet24 and Favalli7 (both 64%) and the British Society of Rheumatology Biologic Register (59%)27.
The eight-year survival rate in our study (56.3%) was longer than Favalli’s study (51.9%), although our results might have less consistency because we started with a very small sample (33 patients) compared with that of Favalli (298 patients).
In our study, the cumulative drug survival proportion of patients at eight years was 85.7%, 60.0%, 52.5%, and 44.4% for Goli, Ifx, Eta, and Ada, respectively. These results do not concur with data reported by Favalli et al. in which the drug survival rate after eight years is 65.8%, 51.8%, and 44.9% for Eta, Ada, and Ifx, respectively. Ifx survival in patients with PsA in our study was longer and Ada shorter. Goli was the drug with the longest survival in both PsA and SpA in our study.
The causes of discontinuation were mostly adverse events (mainly infections) followed by secondary inefficacy and primary inefficacy, in concordance with a previous study performed in our hospital that enroll 531 patients with rheumatic diseases (53.1% with RA and 46.9% with SpA and PsA), which shows that immunogenicity and infections are the most frequent causes of discontinuation28. However, the total percentage of discontinuations (11.7%) is lower than in our study, because the population of our study was smaller, probably.
Favalli et al. report a discontinuation higher than our study (42.08% and 44.63% for SpA and PsA, respectively)7. Discontinuations due to secondary inefficacy are higher in Favalli´s study (32.33% in SpA and 40.60% in PsA). Adverse events (3.00% in SpA and 9.02% in PsA) are lower and similar, respectively. Primary inefficacy is lower in SpA (5.26%) and higher in PsA (9.77%).
Limitations
This study has some limitations, such as the fact that there are no data on the time from diagnosis to the first biological treatment, nor data on the presence or absence of concomitant synthetic disease -modifying anti-rheumatic drugs. Concomitant methotrexate improves drug survival in patients with PsA29 and undifferentiated spondyloarthritis30 and significantly increases the survival of both Ada and Eta in RA19. Another limitation is the small number of patients, especially in PsA, which could affect the consistency of the results. Another limitation is that in the calculated persistence, we have assumed that patients were adherent to the treatment after withdrawing their medication dispensed in the pharmacy service, because this withdrawal does not imply that all patients administer it.
The main contribution of our study is the long study period and the inclusion of all possible biological drugs for the treatment of these rheumatic pathologies. These results add to the results previously published, in which only the short-term or related persistence of the anti-TNF drugs Eta, Ada, and Ifx were studied.
In conclusion, although this study has some limitations, we report that Toci and Eta for RA, and Goli for SpA, and PsA are associated with high long-term persistence and a good profile of safety as first-line treatment. When second-line treatment was prescribed, Toci for RA, and Goli for SpA, and PsA also had a better retention rate than other biological agents. Discontinuation of these drugs in RA was higher than in SpA and PsA (both similar), and the most frequent reason was adverse events in RA and remission in SpA and PsA.
Contribution to the scientific literature
Our study on the persistence of medications for rheumatic conditions contributes with two important aspects to scientific literature: firstly, it is a persistence study, and therefore with health outcomes of biologics at long term, specifically with an eight -year follow-up, both for rheumatoid arthritis and axial spondyloarthritis, radiological (ankylosating spondylitis) and non-radiological, as well as for psoriatic arthritis. The clinical parameters of each condition are inherent to these health outcomes, directly associated with the clinical situation of the patient, as well as drug safety and its good tolerability by patients. Secondly, all medications indicated for these three conditions are included, up to and including the year 2016, unlike most published studies which only include persistence studies for anti-TNF drugs.
Awareness of these data could be useful in our setting, as they can help to make decisions at the time of prescribing biologics at first line based on their persistence over time: considering that all biologic medications for these conditions are equally effective, prescribing those with higher persistence would therefore mean prescribing the more efficient options at long term.