Introduction
The Spanish Royal Decree 1015/2009 (RD) regulated the availability of medications for special situations (MSS)1 and led to two major changes: redefining the uses of some drugs, and modifying the procedure required for their acquisition.
Regarding the redefinition of use, three special situations were clearly defined:
Access to investigational medicinal products for patients outside a clinical trial and without authorised therapeutic alternatives (compassionate use of investigational medicinal products).
The use of drugs under conditions other than those authorised (off-label).
The use of drugs approved in other countries but not in Spain, generally for marketing reasons (i.e. foreign drugs).
Regarding procedural modifications, the need for individual authorisation by the Spanish Agency of Medicines and Medical Devices (AEMPS) on a case-by-case basis was eliminated and transferred to health care centres.
Another special situation refers to drugs that have been approved by the AEMPS for a particular indication, but are not included in the hospital's Pharmacotherapy Guidelines (HPG), and that at the time of request are not included in the HPG for their prescription to patients from a specific autonomous community or patients treated in hospitals.
The work of hospital PTCs2-5 been driven by the need to justify the use of MSSs or drugs not included in the HPG, and to inform patients of potential benefits and risks to obtain their informed consent. In our hospital, this activity was defined in the Spanish RD 86/2015, which creates and regulates the composition, organisation, and running of the PTC in the Balearic Islands6. Blanco-Reina et al.7 and a French consensus document that assessed medicines8 have suggested that the large volume of off-label prescriptions requires follow-up concerning the effectiveness and costs of MSSs not included in the HPG. However, very few studies have assessed the results obtained from the use of these medications in terms of effectiveness, safety, and associated costs9-13.
The main objective of this study was to analyse the clinical response to the use of these drugs and their costs. The secondary objective was to describe the use and type of medications requested by the medical services.
Methods
Retrospective study conducted in a secondary care hospital within a health area serving 265,000 inhabitants. The analysis included drugs for compassionate use, off-label drugs, and drugs not included in the HGP (non-HPG medications) whose use was requested from the hospital PTC from January 2018 to December 2018. Although foreign medications may be classified as MSSs, requests for these drugs were excluded because they are not processed by our hospital's PTC. The follow-up period for each request processed was established according to the expected outcome of the treatments.
In order to comply with the requirements of the RD1,6, we designed Standard Operating Procedures (SOP), which were submitted to and approved by the PTC. According to the SOP, the PTC assumes responsibility for assessing the internal use of off-label, compassionate use, and non-HPG medications. The SOP consists of the following stages:
Creation of the request: the responsible physician makes an electronic request for the patient's new medical treatment. The report includes the following information: medication requested, dosage, indication, treatment outcome expected by the physician, bibliographic references justifying its use, the patient's clinical report, and informed consent.
Assessment of the request: the Pharmacy Service (PS) receives the physician's electronic request for the MSS and prepares a technical report that includes the following: an analysis of the available evidence on indications for the drug requested; alternatives with an approved indication; alternatives with a nonapproved indication, but with greater scientific evidence; analysis of the economic impact; and proposal for authorization/rejection. The report is then submitted for review and assessment by the members of the PTC.
Resolution of requests: each request is assessed during the monthly PTC meeting. Each request includes the pharmacist's technical report and the requesting physician's clinical report. The PTC issues a final decision which, in the event of approval, must be validated by the hospital's Medical Director.
To investigate the clinical response to each treatment, the PS used Microsoft Access 2007 to design a database in which each drug request was recorded. The variables collected were as follows: medical service, drug, indication, type of request, treatment objective, response to treatment based on whether the objective proposed by the physician was achieved or not, and cost. All data were obtained from the electronic medical record and the integrated drug management system of the PS.
Therapeutic success was defined as the response to treatment matching the objective proposed by the physician in their request form. Therapeutic failure was defined as failure to achieve the clinical objective by the date agreed at the PTC meeting.
All data analyses were conducted using SPSS v.23 software. Study variables were described as tabulated data. Continuous variables are expressed as a means and categorical variables are expressed as estimated total percentages and frequencies. The cost analysis was based on the duration of treatment until therapeutic success was achieved or, failing this, on the number of doses administered until treatment was discontinued. The amount entered in the cost analysis was the price paid by the hospital (company selling price - discounts + VAT).
All the data were collected by a researcher. The request underwent external review by the PTC as well as by the pharmacist responsible for the medical service making the request.
Results
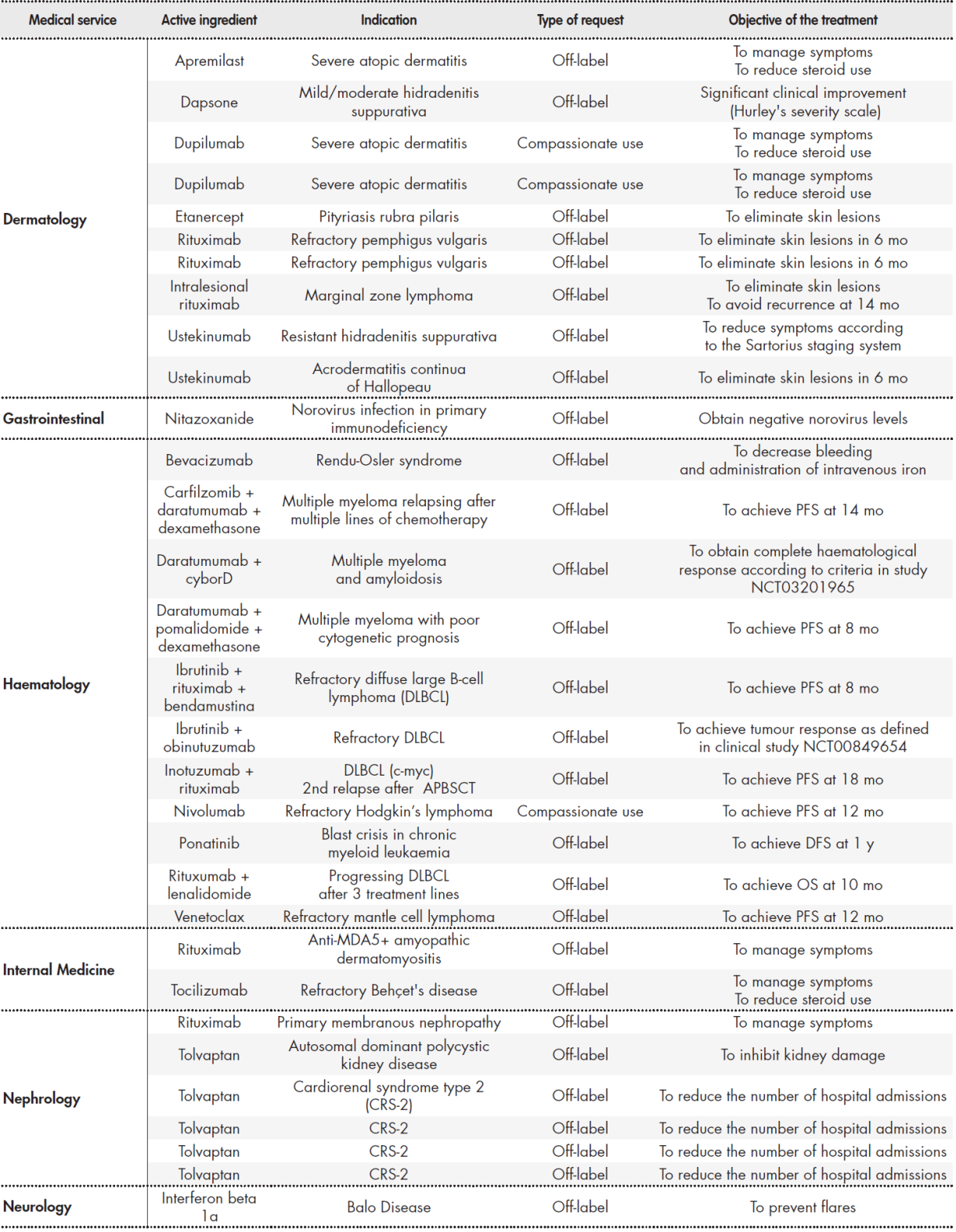
We analysed 70 requests accepted by the PTC during the study period. Table 1 Table 1 cont Table 1 cont shows the requests classified by medical service requesting the MSS. The most requests were made by medical oncology (26 requests), haematology (11), and dermatology (10), which together comprised 67% of all requests. Regarding the type of request, 69% were for off-label drugs, 20% were non-HPG medications, and 11% were requests for compassionate use.
Table 1 (cont.). Requests classified by medical service
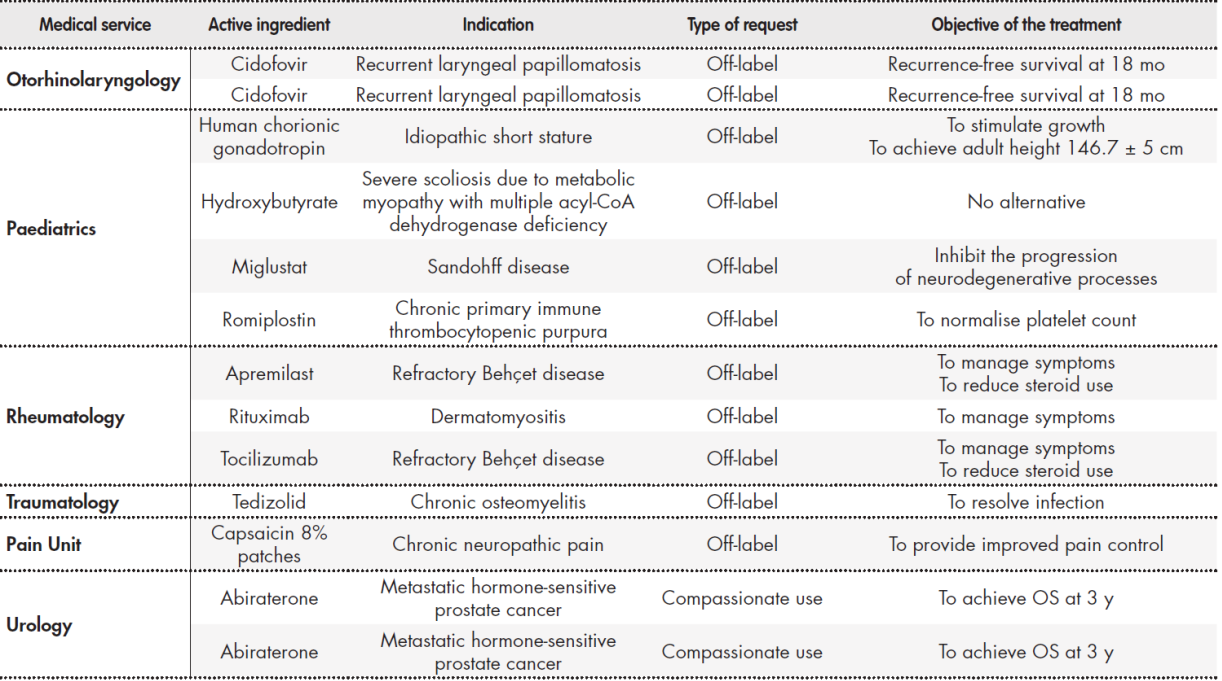
APBSCT: autologous peripheral blood stem cell transplantation; ChT: chemotherapy; DFS: disease free survival; DLBCL: diffuse large B-cell lymphoma; HPG: hospital
Pharmacotherapy Guidelines; OS: overall survival; PFS: progression free survival.
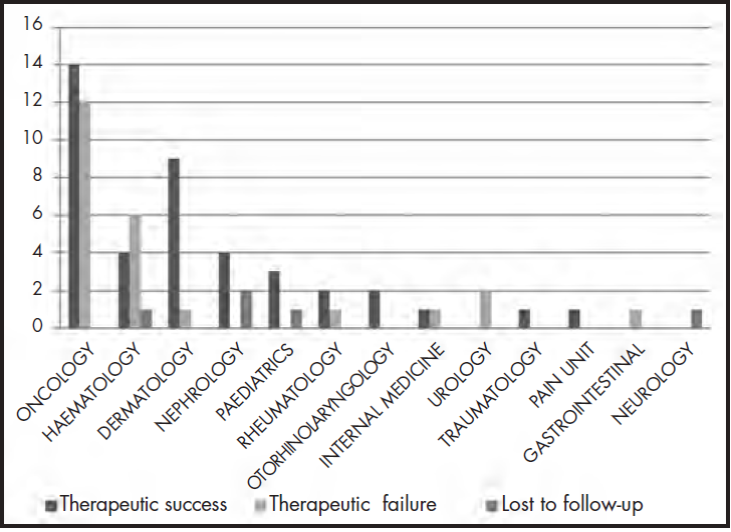
Therapeutic success was achieved in 59% (41 requests) of cases. Therapeutic failure occurred in 34% (24 requests) of cases: that is, the clinical outcomes expected by the physicians were not achieved. In total, 7% of the requests could not be analysed due to loss to follow-up: there were 3 deaths due to causes unrelated to the disease or the treatment received, 1 case in which the patient moved to another autonomous community, making follow-up impossible, and 1 case in which the patient refused to initiate treatment after it had been approved by the PTC. Figure 1 shows the clinical outcomes by medical service requesting the MSS
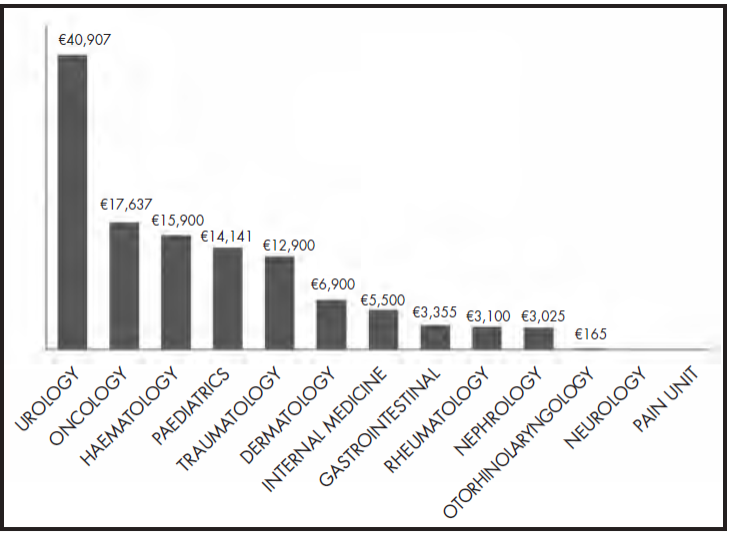
The total cost of the treatments for the 70 requests for special medications and medications not included in HPG wase €1,140,240. The cost of the treatments analysed was €821,631, of which €521,250 (63%) corresponded to therapeutic success and €300,381 corresponded to therapeutic failure (37%). The average cost per treatment request was €16,288. One of the treatments requested by the haematology service, inotuzumab + rituximab (cost = €259,764), was excluded from this calculation in order to avoid distorting the results. Figure 2 shows the cost per request by medical service.
Of the 41 requests in which therapeutic success was achieved, 30 were off-label requests, 4 were non-HPG medications, and 7 were drugs for compassionate use. Of note, 75% of the medications were cytostatic and biologic drugs
Discussion
The assessment of MSSs or non-HPG drugs is a relevant activity, which is currently conducted by hospital PTCs. This study analysed a total of 70 requests approved by our PTC over one year, which is equivalent to almost 6 requests per month.
Off-label prescriptions, the compassionate use of drugs, and the use of non-HPG drugs make it possible for patients to gain benefit from potentially effective treatments. However, off-label and compassionate use also entails a certain level of risk due to the fact that no safety guarantees have been issued by regulatory agencies because the risk-benefit ratios of these therapies have not been analysed for some diseases. The health care system has to invest in treatments for which there is limited evidence concerning their clinical benefits14-16. RD 1015/2009 addressed these aspects and regulated the procedure1 such that it is used under exceptional circumstances and is restricted to situations in which there are no therapeutic alternatives.
Despite these aspects, previous authors have stated that this type of prescribing is a common practice17,18, and is particularly frequent, although for different reasons, in the fields of oncology and paediatrics15,16,19.
There are several situations that may explain the prescription of off-label and non-HPG medications: delays in publishing the results of clinical research and the subsequent authorisation of a new indication by the regulatory agencies; the exclusion from clinical trials of certain groups of patients due to ethical limitations; the lack of interest among manufacturers in registering a new indication; and delays between the time of approval by the AEMPS and the time of approval by the PTCs such that the drugs can be made available in hospitals19-21.
On the other hand, before new and effective but very expensive drugs can be released, the available data needs to be collected and analysed in order to decide as quickly as possible whether these drugs have therapeutic value. The use of MMSs can be improved by monitoring and the creation of records of real-world results. Out of the total number of requests, 59% of the treatments led to therapeutic successes and 7% were lost to follow-up.
It is difficult to compare the results of our study with those of others because of the low number of studies that have assessed the health outcomes of using MSSs and non-HPG drugs.
The multicentre study by Danés et al.9 included the assessment of 232 MSSs for 102 different indications. In relation to therapeutic success, their results were similar to ours: complete response to treatment (31.4%), partial response to treatment (36.3%), and stabilization (4.9%). However, their study addressed different diseases and used different drugs, thus making it impossible to compare the results.
The present study shows that most requests were emitted by the departments of oncology and haematology, which obtained therapeutic success in 49% of cases (18 out of 37 requests). We compared our results to those of a descriptive observational retrospective study conducted by Arroyo Álvarez et al.13, who analysed 154 antineoplastic drugs that had been used in special situations between 2005 and 2015. Regarding treatment, they found a subjective response of 32.5% and an objective response of 10.7%. These authors noted that most of the drugs were used for the treatment of metastatic tumours. Differences in response rates between our study and theirs may be because our study did not include many patients with metastatic tumours.
Our study also shows that the dermatology service emitted the third highest number of requests (10), of which 9 led to therapeutic success. This result is much higher than that obtained by Ong et al.12, who obtained therapeutic success in 70% of the 25 off-label requests from the dermatology service. This difference could be explained by the fact that in the study by Ong et al. 20%, of patients had to discontinue treatment due to adverse events.
Another relevant aspect of the use of MSSs is their economic impact on the health care budget and its sustainability. Our analysis shows that, over the study period, the actual cost of the treatments was more than
€1 million. We compared the average cost per request in our study with that of the study conducted by Arocas Casañ et al.22. Their study assessed the economic impact of 834 requests for MSSs and obtained a cost per request/y of €8,554. In our study, the average cost per request/y was €16,288, which is almost double the figure reported by these authors. This result may be because many of our treatments were administered for more than one year, whereas Arocas Casañ et al. calculated the average annual cost.
In the setting of dermatology, a comparison of our cost per request and that of Ong et al.12 shows that the costs per request/y were €4,348 and €2,755, respectively. There are two reasons for this difference. Firstly, we estimated the cost of the full treatment rather than the yearly cost of the treatment. Secondly, in the study by Ong et al., 20 of the 25 requests were for thalidomide, mycophenolate mofetil, and cyclosporine, which have lower costs than those requested in our study.
When assessing the cost-benefits of the all the treatments assessed, excluding the 7% lost to follow-up, we found that 63% of the budget was spent on treatments that achieved therapeutic success. As Ong et al.12 suggested, the costs of these drugs may be offset by a decrease in the number of times patients are admitted to hospital (i.e. lower morbidity with improved quality of life) and by the use of alternative treatments.
In our hospital, half of the requests were made by the haematology and oncology services and accounted for more than 75% of the budget for MSSs and non-HPG drugs. This result could be due to the upsurge in these areas of research and the rapid dissemination of results from clinical trials before these drugs are approved by the regulatory agencies19.
Of note, there were few requests from the paediatric medical services in our study. The literature suggests that the prescription rate for off-label drugs is more than 50%(23.24). However, only 4 out of the 70 requests assessed by our PTC were destined for paediatric use. This disparity is probably due to our hospital not being a Maternity and Children's hospital. Nevertheless, it could also be due to possible under-reporting to the hospital's PTC.
This study has some limitations. Firstly, the results cannot be generalized. Secondly, extrapolation would be complex because of the heterogeneous diseases and drugs included in the study and the low number of cases in each disease or drug group. Nevertheless, it presents a reliable picture of the situation in our hospital, while increasing our knowledge of the realworld results of the use of these drugs, thus providing the scientific community with evidence on this topic.
The aim of this study was to design a model for managing MSSs and non-HPG medications, while complying with the regulations described in RD 1015/2009 and RD 86/2015. The assessment and monitoring procedure proposed by the multidisciplinary PTC ensures that ethical factors are taken into account during decision-making and that access to treatment is based on best evidence for the patient and cost-effectiveness. Prospective monitoring of the patient during treatment makes it possible to re-evaluate the patient if the objective of the physician is not being achieved. It also makes it possible to measure whether care is providing value, where value is understood as clinical benefit.
In conclusion, in our hospital, more than half of the MSSs and non-HPG medications met their therapeutic objectives, although the economic impact of their use was high.
Contribution to the scientific literature
The prescription of medications for special situations, off-label drugs, or drugs for compassionate use, is a common and widespread practice that allows patients to benefit from a potentially effective treatment. However, this practice entails certain risks, because these drugs are used in conditions in which there is insufficient evidence to guarantee a positive risk-benefit ratio. Thus, their use must be properly explained to patients, who are required to give their informed consent. Experts in medication surveillance strongly recommend strict follow-up and monitoring during these treatments. Despite this, few studies have assessed the health outcomes obtained from the use of these drugs.
The present study analysed a model for managing medications for special situations, such as off-label drugs and drugs not included in our hospital's Pharmacotherapy Guidelines. Requests for the use of these drugs were sent to the hospital's Pharmacy and Therapeutics Committee. The Pharmacy and Therapeutics Committee assessed and monitored the requests to ensure that each treatment was based on best evidence and cost-effectiveness to guarantee that both patients and the health care system obtained benefits. The monitoring and creation of records of real-world results and the appropriate analysis of outcomes can contribute to the development of strategies to improve the rational and reasonable use of these drugs.











 text in
text in