Introduction
COVID-19 is a disease caused by infection with the new SARS-CoV-2 coronavirus, which has led to an unprecedented world pandemic with significant implications for public health, society and the economy1,2. Global efforts to fight the pandemic have consisted in conducting extensive research and developing a vaccine able to prevent the infection. Even since the genetic sequence of SARS-CoV-2 was published on 11 January 2020, over 300 vaccine candidates have been analyzed in clinical or preclinical trials, with vaccines BNT162b2 (Comirnaty®, Pfizer/BioNTech), mRNA-1273 (COVID-19 Vaccine Moderna®), ChAdOx1-S (COVID-19 Vaccine AstraZeneca®) and COVID-19 Vaccine Janssen having been granted conditional marketing authorization by the European Medicines Agency (EMA)3-6.
Up to October 2021, the percentage of people vaccinated with at least one dose rose to 48.7%7 However, low vaccination rates in low-income countries8 and the existence of a significant minority of people who have doubts regarding vaccines against COVID-199 constitute a significant hurdle to effectively controlling the spread of the virus. Recent studies on the acceptance of these vaccines show that fear of side effects is the main reason for choosing not to get the COVID-19 jab10,11. A systematic review if the strategies deployed to tackle people's doubts about vaccines revealed that providing the population with truthful information on potential side effects is vital to improve acceptance12.
Vaccine safety analyses look into whether statistically significantly higher rates of a given adverse event (e.g. fever) are observed in the vaccinated group than in the control group. They also look into factors such as biological plausibility as well as the length of time elapsed between vaccination and the onset of an adverse event. According to the data from initial clinical trials, the most commonly reported adverse reactions by participants vaccinated against COVID-19 include local reactions (e.g. injection site soreness) and systemic reactions (e.g. headache, muscle pain)13-16. Such reactions tend to be transient and of a mild-to-moderate nature17, with severe or lifethreatening adverse reactions being very rare3-6.
The data available to date on the side effects of vaccines against COVID-19 have been published in studies funded by pharmaceutical companies that manufacture them and monitored by third parties. The promotion of independent studies analyzing vaccine safety could favor acceptance by the population, allowing a more effective control of the spread of the virus. Likewise, although pharmacovigilance systems make it possible to characterize the safety profile of vaccines by providing information on adverse reactions, spontaneous reports of such adverse reactions do not provide any insights into the prevalence of those reactions given that the reports are normally made by individual patients who only tend to report significant reactions. On the other hand, systematic monitoring of the local and systemic reactions resulting from the vaccines could be useful to healthcare providers and to the general population alike.
The present study is aimed at examining the reactogenicity of the first and second dose of an mRNA vaccine against COVID-19. Specifically, an analysis was made of the reactogenicity of the BNT162b2 vaccine (PfizerBioNTech) in a group of workers from a third-level hospital of Huelva province. Given that it has been shown that adverse reactions are not evenly distributed among the population, consideration was also given to the factors related to the higher reactogenicity of the vaccine in some cases.
Methods
This was a single-center observational post-authorization safety study. A convenience sample of 305 healthcare and non-healthcare workers from a third-level hospital in Huelva province was obtained to gather information on the local and systemic reactions of mRNA vaccine BNT162b2 against a COVID-19 (Pfizer-BioNTech). All patients had received their first dose of the vaccine between January and March 2021, with the second dose being administered to them 21 days later. All participants were aged between 18 and 65 years. Information was compiled through a questionnaire where participants had to state the local and systemic reactions experienced, if any, within seven days from receiving each jab of the vaccine.
Before administration of each dose, all participants were informed about the characteristics of the study, particularly about the anonymous and voluntary nature of their participation. Written informed consent was obtained from all subjects before their inclusion.
The protocol for this research study was approved by the Biomedical Research Ethics Committee of Andalusia.
Variables
Subject characteristics: The subjects' sex and age were duly recorded. Previous infection with COVID-19: Participants were asked to state whether they had received a confirmed diagnosis of COVID-19 prior to vaccination.
Adverse event questionnaire: Based on the safety information on the NT162b2 vaccine's SmPCs18, a questionnaire was prepared that included a local reaction (injection site soreness) and seven systemic reactions (headache, fever, insomnia, arthralgia or myalgia, nausea, fatigue and general malaise). Moreover, given that the EMA's pharmacovigilance system has identified other les frequent adverse reactions, participants were allowed to state whether they had experienced other reactions.
This analysis of adverse reactions made it possible to: (1) record the prevalence of each of the local and systemic reactions evaluated; and (2) calculate the vaccine's overall reactogenicity based on the sum of all the reactions reported by each participant (range 0-9) following each jab.
Statistical analysis
Descriptive analyses were carried out to characterize the sample and examine the incidence of local and systemic reactions following the first and second dose of the vaccine. McNemar's test was used to evaluate differences in prevalence between the adverse reactions reported following the first and the second dose of the vaccine. Pearson's chi-square test was applied to study the relationship between sex, age and each of the local and systemic reactions evaluated following each dose of the vaccine. Student's paired sample t test was used to evaluate the difference in overall reactogenicity between the first and second dose. Student's t test for independent samples was employed to compare differences in the overall reactogenicity of the first and second dose of the vaccine according to sex, age, and previous COVID-19 infection. Finally, a linear regression model was applied to evaluate the relationship between the overall reactogenicity of the first and second dose, controlling for individual differences and previous infection with COVID-19.
Results
Of the 305 initial participants, 291 filled out the questionnaire after receiving the first and second dose of the vaccine (95.4% response rate). That was definitive size of the study sample. Two-hundred of these 305 subjects were female (68.7%) and mean age was 48.46 [Standard Deviation (SD) = 11.77]. A total of 6.9% of participants declared having been previously diagnosed with COVID-19.
Adverse reactions of the vaccine against COVID-19
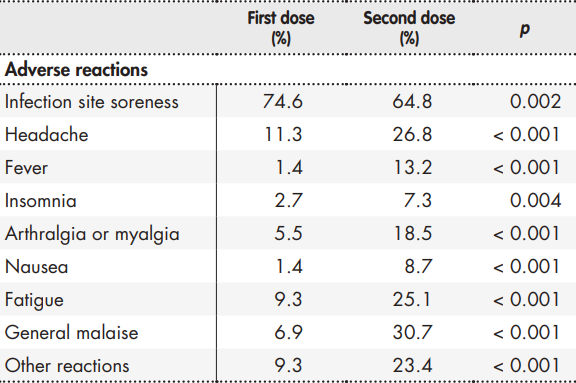
A total of 81.8% and 84.0% of participants, respectively, declared having experienced at least one adverse reaction following the first or the second jab of the vaccine. Table 1 shows the prevalence of adverse reactions following the first and second dose. An analysis of the reactions occurring between the first and the second dose shows that systemic reactions were more common after the second dose, while local reactions were more usual after the first dose.
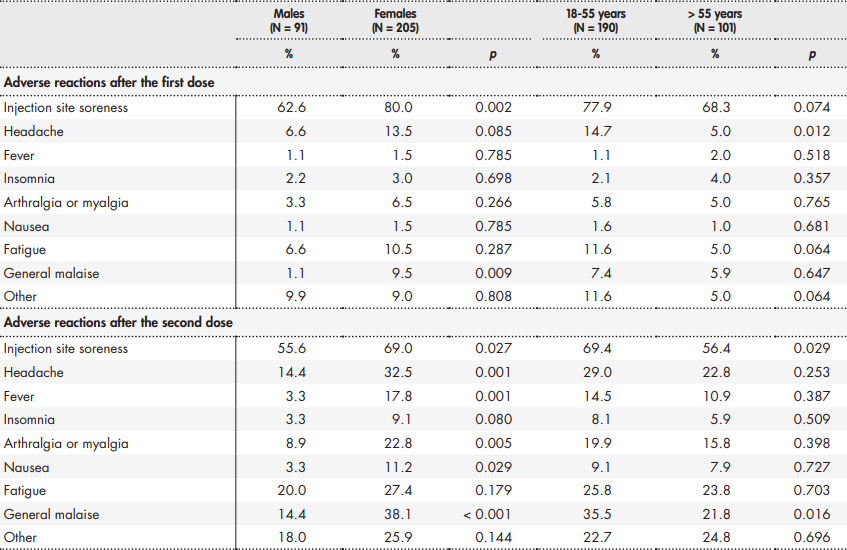
Table 1. Prevalence of adverse reactions following the first and second dose of the BNT162b2 vaccine against COVID-19
Other reactions were also analyzed, yielding a total of 15 different reactions following the first jab and a total of 32 reactions after the second jab. These reactions, all of them all mild, were classified into (1) flue-like reactions (e.g. shivers, throat soreness, sneezing); (2) allergy-like reactions (e.g. rash, cold sores, etc.); (3) gastrointestinal reactions (e.g. diarrhea, vomiting, stomachache, etc.); (4) anxiety-related reactions (e.g. tachycardia, low blood pressure, dizziness, etc.); and (5) other reactions (e.g. impaired vision, nosebleeds, etc.). Most of these reactions appeared in isolated cases, affecting 1 or 2 participants. The most common reaction after the first dose was dizziness (n = 12) and the most common reaction after the second dose was shivering (n = 18).
Table 2 shows the prevalence of adverse reactions as a function of participant sex and age.
Overall reactogenicity of the vaccine
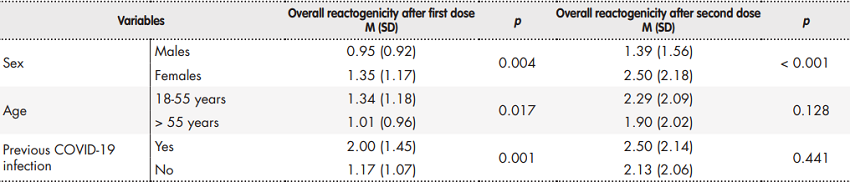
The overall reactogenicity analysis of the vaccine showed that the number of reactions reported by participants after receiving their second jab (M = 2.10; SD = 2.07) was significantly higher than the number of reactions reported after the first jab (M = 1.22; SD = 1.11; p < 0.001).
The vaccine's reactogenicity exhibited differences related to sex, age, and prior infection with COVID-19 (Table 3). Females reported a higher number of reactions than males following both the first (p < 0.05) and second administration (p < 0.001) of the vaccine. As regards age, although subjects between 18 and 55 years of age showed higher reactogenicity levels after the first jab (p < 0.05), no statistically significant differences were found following the second dose of the vaccine (p = 0.128). Participants with a prior COVID-19 infection had higher reactogenicity after the first dose than the resto f participants (p < 0.01) but no statistically significant differences were observed between the two groups following the second dose of the vaccine (p = 0.441). Furthermore, the reactogenicity analysis conducted after administration of the first jab in participants with a previous infection (M = 2.00; SD = 1.45) did not show statistically significant differences (p = 0.784) with respect to the second dose administered to patients with no previous COVID-19 infection (M = 2.13; SD = 2.06).
Finally, when controlling for sex, age and previous COVID-19 infection, it was observed that a high reactogenicity after the first dose was related with higher reactogenicity after the second dose of the vaccine (β = 0.34 [confidence interval 95% = 0.23-0.45], p < 0.001).
Discussion
The purpose of this study was to examine the reactogenicity of a COVID-19 vaccine in a population of both healthcare and non-healthcare workers of a third-level hospital. The findings from the analyses carried out indicated that reactogenicity varied depending on the dose of the vaccine administered (first or second), sex, age, and whether subjects had been previously infected with COVID-19.
The observed incidence of reactions was, on the whole, consistent with the results of the clinical trials and other studies on mRNA vaccines against COVID-193,4,13-16, injection site soreness being the most commonly reported reaction. The next most commonly reported reactions were headache and fatigue following the first jab, and general malaise and headache after the second dose. Moreover, in line with previous studies on the BNT162b2 vaccine14,15, it was found that systemic reactions were reported more frequently after the second dose of the vaccine, while local reactions occurred more commonly after the first dose.
The analysis of local and systemic reactions showed differences as a function of participants' characteristics. As far as gender is concerned. Results showed that females exhibited higher reactogenicity after the first and the second jab. These differences were particularly noteworthy following the second dose, where six of the evaluated reactions occurred predominantly in females. The scarce existing studies analyzing reactogenicity as a function of sex also show a predominance of common reactions in females19,20. Although it seems likely that gender-based differences may be common, no reasons or mechanisms have as yet been proposed to explain this fact. Studies on other vaccines suggest that the sex-reactogenicity relationship is a complex one, influenced by a host of contributing factors such as vaccine formulation, interactions with the immune system, genetic polymorphisms across populations, etc. 21. These potential differences in reactogenicity should be looked into by future studies on COVID-19 vaccines.
Overall, the age-based reactogenicity of the BNT162b2 vaccine was similar to that reported by previous studies15,16. Probably as a result of immunosenescence22, the prevalence of adverse reactions decreased with age, with fewer local and systemic reactions being observed in older adults (> 55 years) than in younger ones (18-55 years). A specific analysis of adverse events indicated that headache following the first dose and injection site soreness and general malaise after the second dose were particularly common among younger adults. As regards the overall reactogenicity of the BNT162b2 vaccine, differences were only observed after the first jab of the vaccine, as a result of the fact that younger adults reported a higher number of adverse reactions. Nonetheless, no statistically significant differences were found between the two age groups following the second dose of the vaccine. In that regard, previous studies on mRNA vaccines against COVID-19 concur that differences between both age groups are also greater after the first dose of the vaccine than after the second one15,16.
The present study found a significantly higher incidence of self-reported adverse events following the first jab of the BNT162b2 vaccine in participants who had had COVID-19 as compared with those without a previous COVID-19 infection. This finding was consistent with previous reports indicating that persons with a previous COVID-19 infection are twice as likely to experience one or more adverse reactions than those without a COVID-19 history 23-25. This may be explained by the fact that individuals who recover from COVID-19 develop a stronger response to SARS-CoV-2 IgG antibodies following one dose of the vaccine than people without a previous infection26, 27. It has also been demonstrated that one single dose of the BNT162b2 vaccine induces a significantly stronger T cell immune response in individuals previously exposed to the virus28. In this respect, it has recently been suggested that following their first dose of the vaccine, the reactogenicity demonstrated by people with a previous COVID-19 infection after the first dose of the vaccine is comparable to that observed following the second dose given that they have already been exposed to the disease24. As part of this study, an analysis was performed of the reactogenicity after the first jab in people with no previous exposure to COVID-19 and after the second jab in people in those who had had the disease, with no significant differences being observed. This finding would seem to support our working hypothesis.
Similarly, no reactogenicity differences were found following the second dose between the groups with and without a previous COVID-19 infection. In this case, all the participants vaccinated with the second dose had been exposed to the viral antigen (either because of a prior infection or when receiving the first jab), with a similar response being elicited in both groups following the second dose. In fact, the response of the SARS-CoV-2 IgG antibodies in people with and without a previous infection after the second dose did not show any significant differences29.
The relationship found in the present study between the reactogenicity of both doses indicates that the people who reported a higher number of reactions following the first dose also did so after the second dose of the vaccine. This finding contributes information that is particularly relevant for monitoring the local and systemic reactions occurring after administration of the second dose. Following up on those individuals who exhibited higher levels of reactogenicity after the first jab would also allow identification of people most likely to develop a higher number of reactions after the second dose of the vaccine.
This study presents with several limitations. Firstly, the information on adverse reactions was gathered using self-report questionnaires, which makes it subjective. Secondly, the presence of biases cannot be ruled out given the low number of participants who reported a previous infection with COVID-19. Another limitation has to do with the fact that the occurrence of reactions was only monitored for up to seven days as reactions following COVID-19 vaccination tend to appear within 24-48 hours as the occurrence of adverse events beyond that period is much less frequent30. Another limitation of this study is the failure to evaluate the intensity of adverse reactions. Lastly, it must be mentioned that no information on the patients' comorbidities was collected and, given that the studied population only included active workers, no data was included on the population older than 65 years or younger than 18 years.
Despite these limitations, the results obtained in this study support the idea that vaccines against COVID-19 are safe in the short term and that there are a few factors related with their reactogenicity. Our wish is that the results obtained may help healthcare providers identify people at a higher risk of experiencing higher reactogenicity levels against COVID-19 so that people receiving the vaccine can be appropriately informed about its potential short-term adverse effects and how to manage them. Moreover, the findings of this study may contribute to a better understanding of the short-term safety profile of the vaccine outside the realm of clinical trials. Conveying this information to the general population may help counteract the negative effects of false or erroneous information about the safety of COVID-19 vaccines.











 texto em
texto em