Introduction
Patient empowerment has become increasingly important in recent years. However, the lack of conceptual clarity and a specific methodology makes it difficult for patients to be included in clinical decision-making 1,2. In the other hand, the limited resources in health systems and a growing need for health care by population make priority setting essential in clinical practice3. Likewise, the World Health Organization has published reports on excessive health care spending in certain clinical areas, such as oncology and hematology4.
How can we take patients' opinions into account and set priorities? The answer to this question could be found both in studies that assess their preferences and in scientific evidence about medicines. The preferences of onco-hematological patients are clear: increased survival, quality of life (QoL), a good death and preservation of dignity5-8. Regarding scientific evidence about medicines, overall survival (OS) and QoL are considered the most appropriate endpoints to assess the benefit received by oncohematological patients9,10. Even though progression-free survival (PFS) is a surrogated endpoint of considerable clinical relevance, this outcome must be carefully analyzed according to the clinical context, and it is not exempt from controversy in many cases11,12.
The European Medicines Agency (EMA) is a participating institution in regulation and monitoring of drugs in the European Union (EU)13. This entity evaluates the benefit provided by novel drugs. Medications must be authorized before being marketed in EU. European system offers different procedures for marketing authorization. Most of drugs are not authorized in EU through a centralized procedure, but are authorized by competent national authorities of the member states. The decentralized procedure allows pharmaceutical companies to request authorization for the simultaneous marketing of a drug in several states without prior authorization in any country. The mutual recognition procedure allows companies with a drug authorized in one state to recognize the authorization in other countries.
On the other hand, centralized procedure makes it possible to market a medicine on the basis of a single European evaluation and a marketing authorization valid throughout the EU. Pharmaceutical companies present a single authorization request to EMA. The Committee for Medicinal Products for Human Use develops a scientific evaluation and makes a recommendation to the European Commission on the marketing authorization. The centralized marketing authorization of the European Commission is valid in all EU states. The use of the centralized procedure is mandatory for some drugs, such as treatments for rare diseases and antitumor therapies. The centralized marketing procedure is a legal requirement that guarantees the efficacy and safety of these drugs. Transparency is an important feature of European system of regulation of medicinal products. A European Public Assessment Report (EPAR) is published for each drug which a marketing authorisation is granted or refused following assessment of EMA.
Randomized clinical trials (RCTs) are the studies with the highest level of scientific evidence, becoming the most robust tool for analysis of health interventions14,15. However, evaluating agencies are often forced to position therapeutic alternatives or authorize them with minor investigations, such as retrospective descriptive studies16. The demand by pharmaceutical industry and patient associations for greater acceleration of drug approval processes could favour decision-making with premature data, increasing the degree of uncertainty regarding them. This could have notable consequences on effectiveness, safety and efficiency of authorized treatments, especially in onco-hematological pathologies.
Taking all of above into account, we can deduce that it is not easy to satisfy the needs of patients in the current health-economic context. Health professionals and government institutions have a common responsibility: to provide the population with the best health care available by optimizing resources. For this reason, studies that analyse the demands of patients are an enriching source of information for health systems, and could improve drug selection. There are numerous validated tools to meet the expectations of cancer patients. Trask et al. developed a 16-item patient-reported questionnaire to evaluate cancer patients' experiences17. This survey contains information about the expectations of effect of antitumor therapy on increased OS. However, this work does not provide information on whether patients expect treatment to improve QoL or patients' preferences between OS and QoL. Rose et al. evaluated patients' care preferences and opinions of doctors with a questionnaire18. In this case, the preferences of patients between OS and QoL were analyzed. On the other hand, the perspective of doctors on the OS and QoL of patients is considered. However, patients were not questioned about their expectations in the therapies received. Gleason et al. tested relationship between cancer patients' expectations for cure prior to interacting with their oncologist and their decisions to follow treatment recommendations19. This study evaluated patients' expectations about the effect of treatments on their cure –which was not exactly the increase in OS– or QoL. However, this questionnaire did not report data on patient preferences on the choice of OS or QoL.
The development of a study encompassing the information of the cited tools could provide interesting information. The objective of our study is to describe the expectations and preferences of our onco-hematological outpatients treated with oral drugs, and to assess the agreement with the results described in EPARs.
Methods
Based on previous literature about preferences of onco-hematological patients5-8, a survey was developed to collect the information of outpatients diagnosed with a neoplasm in our healthcare center. This tool was designed to record expectations and preferences of patients about their treatments, in order to compare them subsequently with results of final endpoints —OS and QoL— presented in EPARs13. The questionnaire presented an initial explanation to inform patients about the anonymity and voluntary participation, and it was divided into two parts. In the first part, clinical and sociodemographic variables (age, gender and clinical context of the participants) were recorded. The second part consisted of three items: (I) patients' expectations about the benefit obtained by their treatment, (II) patients' preferences about the possible benefits that a treatment can contribute and (III) willingness to receive novel treatments with non-definitive results. The recruitment of participants was developed by two hospital pharmacists in the outpatient dispensing area between January 2020 and March 2020. The tool was given to patients, who were also reported on the possibility of requesting for pertinent explanations in case of doubts during the process. Subsequently, participants were surveyed with the necessary time and privacy. Figure 1 shows the questionnaire developed on the recruited outpatients.

Figure 1. Questionnaire about expectations and preferences of onco-hematological outpatients re-garding the treatments for their pathology
Furthermore, a search was conducted of the first EPAR published about the drug received by each patient, in order to analyze results of OS and QoL in the corresponding indication. The following data were registered: date of report, study design, comparators, magnitude of effect of treatments, hazard ratio (HR), confidence intervals (CI), and statistical significance (p). The drug was assumed to provide benefit in OS respect to the comparator when statistically significant difference in OS medians was observed. Benefit in QoL of a drug was considered when a statistically significant difference was demonstrated in any of analyzed scales respect to the comparator. Non-randomized studies without control arm were excluded due to their significant biases. The main limitation of these studies is the difficulty of establishing a causal inference of effect of treatments14,15. The review of reports was conducted by three hospital pharmacists.
Subsequently, an analysis was developed to determine the agreement between the survey items I and II (expectations and preferences on the benefit of treatments) and the results reported in EPARs, in terms of OS and QoL. For this purpose, kappa index (κ) with its 95% confidence interval (95%CI) was used, according to the following formula: κ = [Observed agreement (Ao) – Expected agreement (Ae)] / (1 – Ae). Ao was defined as the most agreed-upon response, and Ae was the expected agreement according to the number of possible responses for each question. Landis and Koch criteria were followed to interpret the strength of agreement for κ values20: < 0.0 was related to non-agreement, < 0.2 insignificant agreement, 0.21-0.4 discrete agreement, 0.41-0.6 moderate agreement, 0.61-0.8 substantial agreement and 0.81-1 almost perfect agreement. The responses of patients to question III were compared with the results of EPARs to evaluate their willingness and exigencies to receive novel treatments with non-definitive results, that showed uncertain benefit in OS or QoL. The Ao value was used to determine the agreement, due to it was not possible to calculate κ. For patients who were willing to receive these novel treatments regardless of data from EPAR it was assumed that they did not have exigencies. However, patients who were not willing to receive these treatments were considered to present demands. In this group of patients, those who were treated with drugs associated with benefit in OS or QoL were considered to meet their exigencies; those users who were treated with drugs without benefit did not meet their exigencies. All calculations were performed using SPSS® v.18 statistical program and p < 0.05 value was considered as statistically significant.
Results
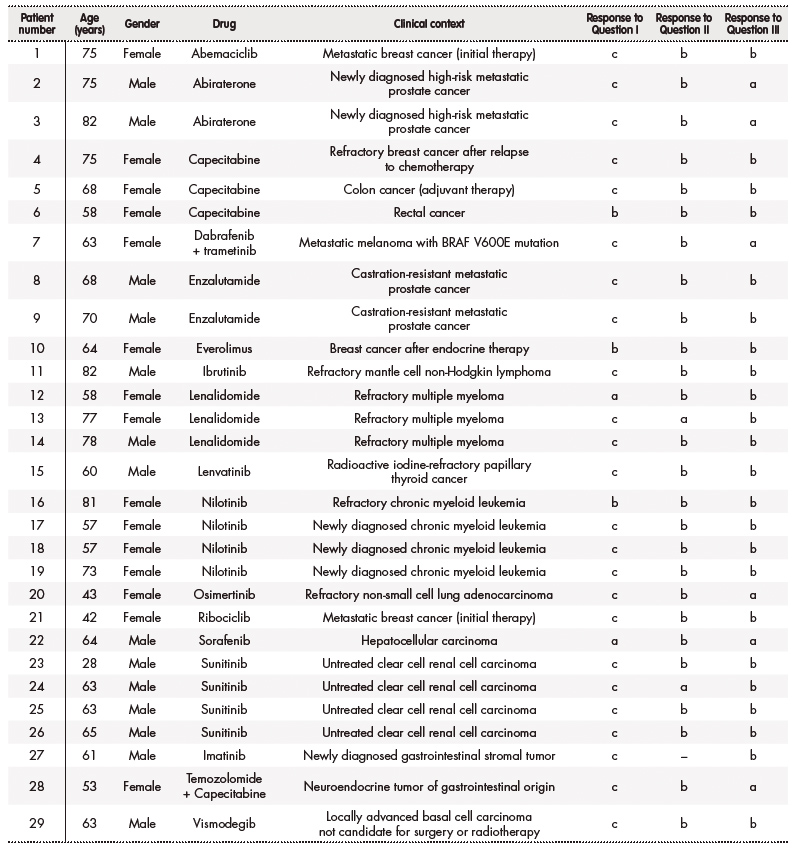
There were 29 participants in the survey, 15 (51.7%) were women and 14 (48.3%) were men. Median age of patients was 64 (28-75) years. The distribution of registered treatments was: 4 (13.8%) nilotinib, 3 (10.3%) sunitinib, 3 (10.3%) lenalidomide, 3 (10.3%) capecitabine, 2 (6.9%) abiraterone, 2 (6.9%) enzalutamide and 12 (41.4%) others. The clinical contexts of participating patients were the following: 4 (13.8%) newly diagnosed clear cell renal cell carcinoma, 4 (13.8%) metastatic castration-resistant prostate cancer, 3 (10.3%) refractory multiple myeloma, 3 (10.3%) newly diagnosed chronic myeloid leukemia, 2 (6.9%) newly diagnosed breast cancer, 2 (6.9%) refractory breast cancer and 13 (37.9 %) others. Data of patients are detailed in table 1.
A total of 19 different indications were registered. There were patients who received the same treatment in the same indication. The EPARs evaluated the following drugs21: abemaciclib, abiraterone, capecitabine, dabrafenib associated with trametinib, enzalutamide, everolimus, ibrutinib, lenalidomide, lenvatinib, nilotinib, osimertinib, ribociclib, sorafenib, sunitinib, imatinib and vismodegib. The publication dates of reports were between 2006 and 2018. Designs of studies included in reports were: superiority RCT in 12 (63.2%) cases, non-inferiority RCT in 3 (15.8%) and 4 (21%) non-randomized studies without control arm. Placebo was the comparator in 8 (42.1%) studies. Comparative OS data were available in 15 (78.9%) indications, while comparative QoL data were available in 6 (31.6%). Individual results of EPARs consulted are shown in table 2.
Table 2 (cont.). Individual results of consulted European Public Assessment Reports (EPARs)

†The standard schemes associated with the novel drug and comparator were not detailed to simplify the information in table.
‡HR: hazard ratio.
OS: overall survival.
%CI: confidence interval percentage.
§QoL: quality of life.
¶The acronyms of this column correspond to the names of different scales analyzed in studies.
ꝢNR: not reached.
¥Confidence interval percentage: 97.5%.
Patients' expectations about the drug (question I), results of preferences about the benefit obtained by a treatment (question II) and willingness to receive a novel treatment with uncertain improvement in survival or QoL (question III) can be consulted in table 3. One patient did not answer the question II. One patient was treated with a combination of drugs –capecitabine associated with temozolomide– without an indication authorized by EMA, therefore the responses to questions I, II and III could not be included in the analysis in this case. Individual responses of each patient to the questionnaire can be found in table 1.
According to willingness of patients to receive a novel treatment with uncertain benefit in survival or QoL (question III), results of EPARs met the requirements of 10 (43.5%) participants with exigencies, while not in 13 (56.5%) of these patients. If the total number of patients is considered (N = 28), 13 (46.4%) patients did not meet their requirements to access a novel treatment without confirmatory data for improvement in OS or QoL. Table 3A describes overall results of participants' responses and EPARs respect to questions and answers of the survey.
According to estimated κ values, insignificant concordance was observed between results of EPAR and patients' responses about their expectations on the drugs (question I) and preferences of the benefit obtained by a treatment (question II). Ao = 53.6% was calculated between patients' responses and results of EPAR for patients' willingness to receive a novel treatment with uncertain improvement in survival or QoL (question III). Ao values and concordances between patient responses and EPAR are represented in table 3B.
Table 3. Results of statistical analysis
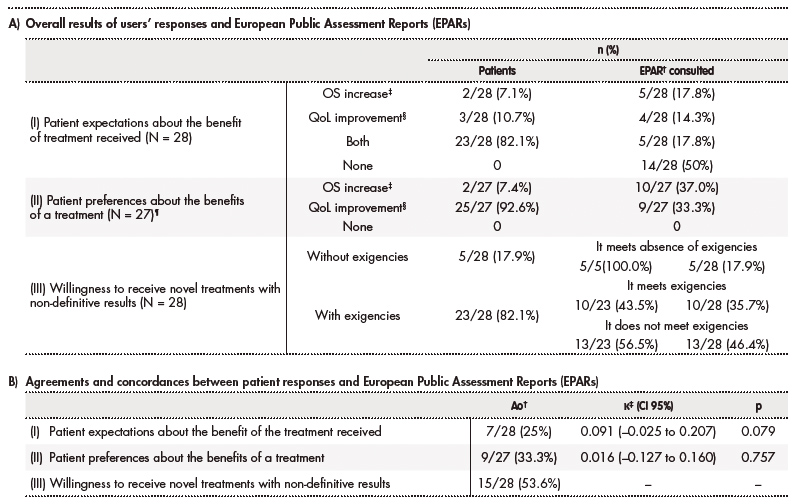
A) †EPAR: European Public Assessment Report. The total number of EPARs consulted was considered equal to the number of patients (N) for each question: 28 EPARs for questions I and III; 27 EPARs for question II. There are patients with the same treatment indication and therefore share the same EPAR (19 different indications).
‡OS: overall survival.
§QoL: quality of life.
¶The results of EPARs were: 5 increased survival, 4 improved quality of life, 5 increased both quality of life and survival, and 13 did not increase either aspect. This is the reason about percentage sum of EPARs is not equal to 100%.
B) †Ao = observed agreement.
‡κ = kappa value.
Discussion
The criteria for marketing authorization of drugs by the EMA are described through EPARs. According to our study, the results of final endpoints described in these EPARs do not fully meet expectations and preferences of our onco-hematological outpatients. The opinion of patients on their treatment should be one of the basic pillars in selection of treatments. Thus, empowerment of patients in clinical decision making would be favoured.
In this work, OS and QoL were the endpoints selected to assess the efficacy of treatments described in EPARs, according to patients' preferences5-8. These endpoints are the most relevant for onco-hematological patients9,10. PFS is also important because it could represent a good indicator of response to treatment. However, we excluded PFS to assess the efficacy of treatments because its interpretation may present a higher degree of subjectivity than the selected endpoints, since it depends on multiple factors such as research center, progression criteria, etc. Likewise, clinical contexts with a doubtful correlation between PFS and OS were described, requiring in-depth analysis. For example, this finding was observed in lung and ovarian cancer studies12,22. Moreover, the understanding of PFS by patients with high age or low sociocultural level could be limited when completing our survey.
The criterion we established for considering the benefit in both OS and QoL was the statistically significant difference between intervention and control arms. For the development of this study, it would have been reasonable to assess the clinical relevance of treatment effect. However, we decided not to contemplate clinical relevance due to heterogeneity of analysed pathologies, controversy about establishing a limit of clinical relevance and lack of consensus in some clinical contexts. Adding the concept of clinical relevance could decrease the number of treatments with positive evaluation in terms of OS and/or QoL, showing a minor agreement between patient opinions and results of EPARs. Moreover, the use of suboptimal treatments or placebo as control arm in RCTs, instead of active treatments, could have a possible influence on the results23,24. The absence of head-to-head trials and indirect comparisons in EPARs was another limitation in the evaluation of benefit of new treatments against the gold standards. Furthermore, it was not assumed that non-randomized studies were able to demonstrate the benefit associated with a treatment. In studies with this design, it is difficult to discern the influence on results of disease, population baseline characteristics and other variables14,15.
Despite not applying the criterion of clinical relevance, almost half of our onco-hematological outpatients received a treatment without benefit in OS or QoL according to EPARs from EMA. The absence of statistically significant difference in OS of novel drugs respect to their comparators is understandable in early clinical contexts or patients with insufficient follow-up25. However, the absence of benefit in QoL of treatments authorized by EMA is hardly justifiable considering the importance of this endpoint26. Almost all our patients preferred an improvement of QoL rather than increase in survival. This finding has already been observed in previous studies6.
Notwithstanding the enormous economic impact of onco-hematological treatments4, EMA does not evaluate the costs associated to treatments. For this reason, it seems reasonable for EPARs to precisely delimit the benefit of novel drugs compared to therapeutic alternatives in a specific clinical context. In this regard, the design of clinical studies that evaluate drugs is of paramount importance. The accelerated access to medicines considering premature results, non-randomized studies or whose validity is not clear, could increase the uncertainty of the benefit-risk ratio. This fact has been verified in the revocation of marketing authorisation for olaratumab associated with doxorubicin in soft tissue sarcoma27 or in the authorization of osimertinib in lung cancer, using non-randomized studies –when there were already approved alternatives with RCTs–28.
A critical analysis of scientific evidence by different health professionals in multidisciplinary committees, also considering the individual opinions of patients, could favour optimization of the drugs selection. We have also observed that almost half of our onco-hematological outpatients would not have been willing to receive their treatment as their requirements had not been met in EMA authorization criteria. Finally, taking all this information into account during the treatment selection process could contribute to patient empowerment.
Our study has several limitations. The questionnaire used was not a validated tool. However, no questionnaires were found that would allow us to collect all the information necessary to develop our study. The selection of individual questions from different questionnaires was not accepted as a feasible option17-19. Therefore, it was necessary to design a new questionnaire. Another limitation of our research was the sample size. The results obtained in this work should be confirmed in investigations with a larger number of patients. Patient selection, time since diagnosis, duration of current treatment, and line of treatment are factors influencing outcomes29. Our study provides individual data on situation of patients (naive or refractory). However, data on the treatment line and time since diagnosis could not be recovered due to the appearance of the COVID-19 health emergency, anonymization of patients and logistical problems. This work could be a pilot study with preliminary results as support for multicenter future research with validation of tools used, larger sample sizes and better selection of patients with criteria according to disease stages, type of neoplasms, time since diagnosis and lines of treatment received.
In conclusion, this study found little agreement between expectations and preferences of our onco-hematological outpatients regarding their oncological treatment and results described in the EPARs from EMA, considering OS and QoL endpoints. Almost half of our onco-hematological participants would not meet their requirements to receive their drug when it was authorized. Other studies should be developed to contrast the results observed in this work.











 texto em
texto em