Introduction
Hypersensitivity reactions (HRs) to chemotherapeutic agents are difficult to predict. They may be classified into mild/moderate events and severe reactions (anaphylaxis)1. The former may be addressed by reintroducing the treatment using slow infusion and premedication. Severe reactions can be managed in two different ways: selecting a different therapeutic alternative or implementing a desensitization protocol (DP).
Discontinuation of a treatment because of an HR may be controversial as it may deprive the patient of a valuable therapeutic alternative. This would be detrimental from a clinical point of view, particularly for patients with few treatment options or with an unfavorable prognosis. Desensitization is a technique that provides patients with temporary tolerance to a drug they are allergic to by progressive administration of increasing amounts until therapeutic doses are reached2. Some patients, however, are refractory to conventional DPs. For these patients changes in premedication are required. Immunomodulatory drugs may be a very useful tool, particularly those indicated for conditions regulated by mediators of HRs3.
Omalizumab is a monoclonal antibody that binds to immunoglobulin E (IgE), reducing the amount of IgE available to trigger the allergic cascade4. It is indicated for conditions such as allergic asthma, hives and chronic rhinosinusitis3. The literature on the off-label use of omalizumab as premedication is scarce, limited to cases refractory to certain kinds of treatment such as platinum-based chemotherapy. The purpose of this case report is to describe the development and administration of a trastuzumab-based DP with omalizumab as premedication in a patient with breast cancer who was refractory to previous desensitization therapy.
Description of the case
This case reports on a 42-year-old woman diagnosed in 2014 with hormone receptor negative and HER2 positive ductal breast carcinoma. She presented with allergic rhinitis as underlying condition. Following diagnosis, she was administered neoadjuvant therapy, which consisted in four cycles of docetaxel-cyclophosphamide, plus two cycles of docetaxel-trastuzumab. During this treatment, she developed an infusion-related reaction to trastuzumab that resolved with antihistamines. A radical mastectomy was performed, after which trastuzumab-based adjuvant treatment was initiated with no complications. In 2019, on detecting lymph node recurrence, an eight-cycle treatment with pertuzumab-trastuzumab-docetaxel was indicated. Following the first cycle, docetaxel had to be withdrawn due to moderate toxicity (asthenia and grade 2 diarrhea). During the third cycle, the patient reported itching and erythema following infusion of trastuzumab. A sensitization test carried out by the Department of Allergology (DA) returned a negative result. A decision was made for the next administration to be based on dexchlorpherinamine with methylprednisolone as premedication. Half an hour into the infusion, a more significant skin reaction than the previous one was observed as well as coughing and an intensification of her underlying allergic rhinitis, which made it necessary to discontinue treatment. As a result of that reaction, the patient was referred once again to the DA for a new DP.
The DA suggested including omalizumab in the new treatment protocol. The Pharmacy Department (PD) developed an analysis of the risk-benefit ratio based on the scarce evidence available. A DP was implemented, which comprised premedication with omalizumab (Table 1) followed by 12 administrations of trastuzumab at increasing infusion rates and concentrations (Table 2), performed in the Intensive Care Unit. During the first and second desensitization cycles, the patient developed an extensive maculopapular rash, which made it necessary to discontinue the infusion. Changes in the premedication were made for the three next cycles (Table 1), which allowed completion of the treatment without further interruptions. When this study was submitted for publication, the patient had received a total of eight cycles —five with a DP— and exhibited a satisfactory response to the treatment.
Table 1. Premedication used in the desensitization protocol
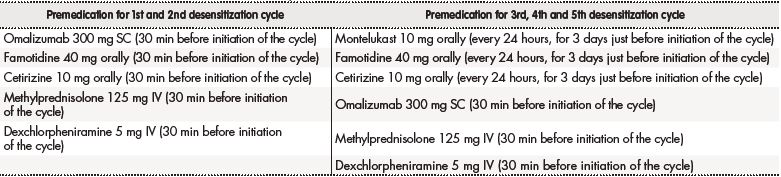
IV: intravenous route; mg: milligrams; min: minutes; SC: subcutaneous route.
Discussion
Our trastuzumab-based DP compounded with omalizumab —and other agents— as premedication allowed safe administration of trastuzumab in a patient with breast cancer who had developed trastuzumab hypersensitivity refractory to desensitization therapy. The 12-step DP with omalizumab as premedication was applied following a specific request by the DA on the basis on previous skin tests and an attempt to reintroduce the treatment without omalizumab. The request by the DA for omalizumab to be included as premedication was based on the type of post-infusion reaction experienced by the patient and on a careful risk analysis. At the beginning, there was certain controversy as omalizumab's SmPC does not contain an indication for the use proposed in this case3. Given the exceptionality of the situation and in line with the hospital's regulations, the Pharmacy Department informed the DA that they had to file a petition requesting the hospital management for authorization to use the drug outside its approved indication. Little information exists, all of it based on case reports, about the use of omalizumab in protocols aimed at desensitizing patients to chemotherapeutic agents (oxaliplatin and carboplatin)5,6. These reports describe the situation of patients who are refractory to desensitization regimens not containing omalizumab, such as the case of our patient8. They all implement DPs that include omalizumab. The case presented here shows how omalizumab can contribute to a DP in association with a drug such as trastuzumab, which had not been part of the regimens reported so far. However, there are cases where it is not possible to evaluate the effectiveness of desensitization either because of subsequent progression of the tumor, which was not the case in our patient, or because a subjective interpretation of the lower intensity of HRs4,5. It must be noted that previous reports on the subject are characterized by a certain variability regarding the omalizumab dose used and how long before the start of the DP it was initiated. In the case of the patient described in this study, it was necessary to combine omalizumab with montelukast, famotidine and cetirizine several days before the start of the DP to ensure an appropriate effect. Moreover, some authors have used omalizumab as desensitization therapy to other kinds of drugs such as aspirin and to foodstuffs such as milk protein8,9.
The development of the present trastuzumab DP was possible thanks to a track record of close collaboration between our pharmacy department and the DA10. Despite the limited information available and the difficulties inherent in associating the inclusion of omalizumab with improved tolerance, this monoclonal antibody could play a useful role in DPs administered to hemato-oncologic patients who have been refractory to previous desensitization therapy to make the most of the therapeutic lines available, as was the case with our patient. Further research is required on large patient samples and with higher levels of evidence in order to confirm the effect of omalizumab in these cases. Collecting experiences from other centers would also be extremely useful. This case report shows that multidisciplinary collaboration among different healthcare providers is essential to improve the safety profile of chemotherapeutic agents.











 texto en
texto en 



