INTRODUCTION
Neuropathic pain (NP) is challenging to manage. It typically results from nociceptive (potentially tissue damaging) neuron hyperexcitability as a consequence of infection, metabolic abnormalities, nerve compression or trauma that leads to neuroinflammation and subsequent spontaneous ectopic activity 1,2,3. In neuropathic pain, the nerve fibers may be damaged, dysfunctional, or injured. These damaged nerve fibers send incorrect signals to other pain centers. A change in structure or chemistry of neurons underlies the production of the altered sensitivity, which is a characteristic of this type of pain. It is relatively common and occurs in up to 5 % of the population. Although numerous treatment options are available for relieving neuropathic pain, there is no consensus on the most appropriate scheme 4,5,6,7,8. Neuropathic pain is often refractory to conventional analgesic regimens and currently there are no drugs that can treat it in a complete and definitive way. Drugs and/or techniques schemes used for relieving NP, usually in a multimodal approach, provide only temporary relief of pain and some are not effective at all. Further, many such medications have serious side effects and some can lead to serious disability to the patient 2,8. Finally, none of these "conventional protocols" provide permanent pain relief, since none promotes the healing process of dysfunctional nerves, being merely conservative and/or palliative therapeutic strategies. Thus, there is no current "effective" evidence-based scheme in treating NP. Recent in-vitro and animal research has demonstrated that platelet-rich plasma (PRP) and/or stem cells therapy could play an important role in the treatment of neuropathic pain, examining the hypothesis that applying this cell-based therapy to the site at which the pain originates can trigger the complete cascade of events involved in tissue repair, leading thereby to the permanent elimination of peripheral neuropathic pain 9,10,11,12,13,14,15,16,17,18. Based on the knowledge that NP is associated with neuroinflammation and ectopic activity as previously pointed out, and understanding that PRP possesses remarkable anti-inflammatory properties and has the ability of promoting axonal tissue repair and regeneration, thus stopping the spontaneous electrical activity and ceasing hyperexcitability (thereby eliminating neuropathic pain), we have conducted a clinical study in which PRP was used as a therapeutic option for the treatment of peripheral NP. The aim of our study was therefore to gain further information about the benefits of platelet-rich-plasma for relieving chronic neuropathic pain.
PATIENTS AND METHOD
This type of research is a prospective study done at the Pain Unit of our institution, in which 45 patients were included. All patients were referred to our Pain Unit with the diagnosis of peripheral NP, with a previous multimodal pharmacological scheme lasting more than 3 months but with poor results. With the preliminary knowledge of the current management of NP above exposed, we presented this novel proposal of interventionist PRP management to the medical ethics committee of the hospital corporation, getting a positive approval.
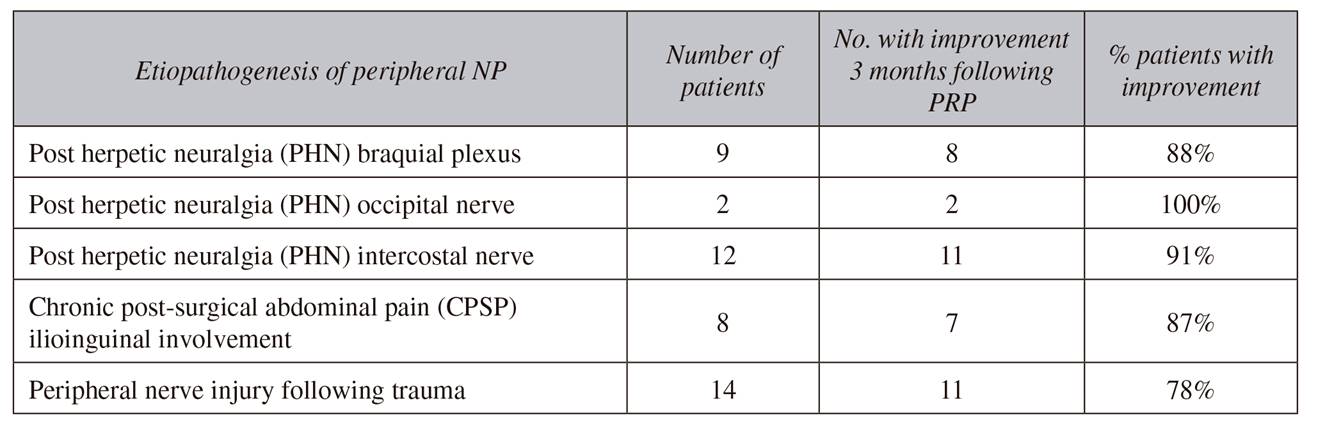
Between June 2015 and December 2016, forty-five patients that arrived to the Pain Unit with the diagnosis of peripheral NP were included in the study. Clinical course of the patients consisted in burning, electric, or lancinating pain in a specific metameric area, most of them with allodynia associated. NP diagnostic criteria used in our unit are stated on the patient's description of pain, that is, a clinical diagnosis. Etiopathogenesis of the peripheral NP included (Table 1): 23 patients with post herpetic neuralgia (PHN) affecting: the braquial plexus (9 patients), the occipital nerve (2 patients) or the intercostal nerve (12 patients); 14 patients with peripheral nerve injury following trauma, and 8 patients with chronic post-surgical pain (CPSP) following abdominal surgery but with clear clinical involvement of the ilioinguinal nerve. Prior to inclusion in the PRP study, all patients had been receiving pharmacologic management for more than 3 months, including tricyclic antidepressants, pregabalin or carbamazepine, and opioids (tramadol, hydrocodone, or oxycodone), in a multimodal regimen, with poor pain relief (EVA pain scale > 7) but with burdensome adverse effects associated to the pharmacological regime. Before to scheduling this novel cell-based procedure with PRP, all patients were explained about the etiopathogenesis of their NP and the probable causes of pain, and about the intended goal with the pharmacological treatment that they were currently receiving (multimodal analgesia). They were also explained of the novel option of PRP therapy and the purpose and expectations with this new technique. All patients signed an informed consent document.
In patients with PHN affecting the brachial plexus, an ultrasound-guided (US) intescalene braquial plexus approach was performed, and 15 ml PRP was injected, spreading the PRP anterior and posterior to the nerve structures and surround the nerves. Patients with diagnosis of ilioinguinal NP received a sonographic-guided PRP injection of 15 ml, considering a good technique when there was a clear separation of the fasciae between de transverse abdominal muscle and the internal oblique. Patients with involvement of peripheral nerves were managed with US approach to the nerve at the site of trauma, and a volume of 10 ml PRP was injected. Patients underwent clinical assessments at baseline, one week, and one, two, and three months after the procedure. Outcome assessed the reduction in pain intensity measured by the visual analogue scale (VAS) which provide data only on pain intensity 19 and the revised "short-form McGill Pain Questionnaire-2" (SF-MPQ-2) 20, a four-factor structure that includes continuous, intermittent, neuropathic, and affective descriptors of pain (the quality of pain is desirable for this type of research), but modifying the response format to a 0-10 numerical rating scale. Daily dosage requirements of the commonly neuropathic-pain medication taken by the patient were also assessed.
RESULTS
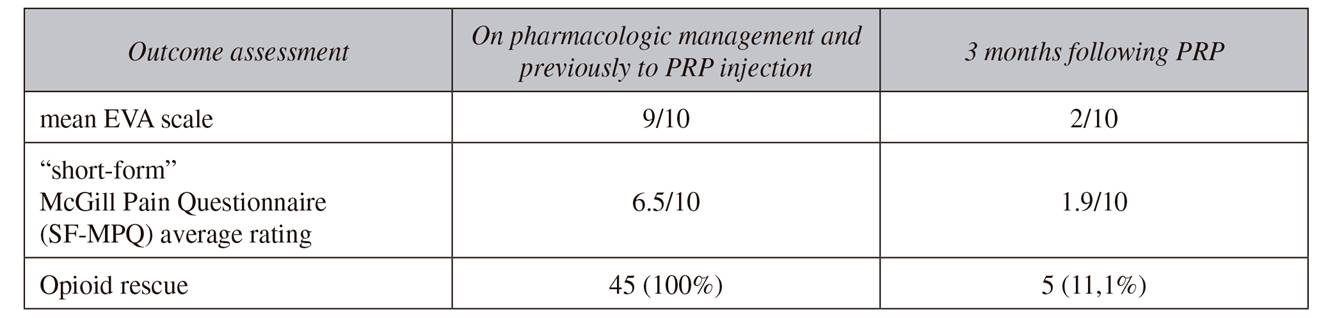
The treatment improved pain and function in 39 out of the 45 patients after the first therapeutic injection of platelet rich plasma. On VAS scale, pain was reduced by 50 % one month following PRP injection and 70 % at the end of three months, scaling down from 9/10 to 2/10. Half of patients (20 patients) reported complete resolution of symptoms and were able to discontinue the use of all pain medication, and opioid use was also withdrawn in another 15 patients, in whom pain control was possible with paracetamol and pregabalin alone. As a consequence of the reduction of pain intensity, patients were able to reduce doses of pain medication, thereby reducing the side effects of the drugs and thus improving their quality of life. However, six patients did not show significant improvement on VAS or SF-MPQ after first dose of PRP and therefore they had to continue the previous therapeutic regimen. However, there were not disclosed complications related to the injection of PRP, during procedure or during follow-up. Characteristics of the patients are shown in Table 1 and Table 2.
DISCUSSION
The International Association for the Study of Pain (IASP) defined neuropathic pain (NP) as "pain initiated or caused by a primary lesion or dysfunction of the nervous system" 21. All neuropathic pain disorders have a common denominator, which is the damage of the somatosensory nervous system: nerve lesions leads to dramatic changes in the nervous system, which makes it different from other chronic pain types that have an intact nociceptive way 22,23. However, the underlying etiologies and pathogeneses of these damages are distinct and not one but several mechanisms can lead to neuropathic pain. Importantly, many of these mechanisms leading to neuropathic pain do not depend on the cause of the disease, as the same mechanism can be found in different diseases. Thus, a general rule in understanding the different mechanisms relevant to NP is that nervous system lesions giving rise to NP involve nociceptive pathways and, although this rule admits exceptions, it is generally valuable and useful in clinical practice 3). Pain due to lesions of the nervous system (whether peripheral, spinal or encephalic) has similar clinical features and shares common mechanisms. In our opinion, the basic and important concept in understanding of the etiopathogenesis of NP and the subsequent treatment proposals that may arise is the consideration that all types of central and peripheral neural lesions, whether ischemic, traumatic, infectious, metabolic, malignant, toxic or immune mediated, are associated to an inflammatory reaction, with increased activity of neutrophils and macrophages that rapidly invade the injured axons and dorsal root ganglion 3,24,25,26,27,28,29,30. Most of the time, such inflammation has neurotrophic and neuroprotective effects but this inflammatory activity can also result in neuronal damage that could contribute to the persistence of NP 26,28. In this context, in the spine, the neuroinflammatory process is associated with the formation of a glial scar and degeneration of the ascending and descending tracts, entailing a supraspinal reactive phenomenon that limits axonal regeneration. In a similar way, peripheral nerve injuries also result in receptor changes in second-order neurons, with spontaneous activity evident in both injured and neighbouring uninjured nociceptive afferents, the so-called central sensitization concept.
Mechanisms associated to NP and evidence-based contribution of PRP to relieving it
Alterations of the Gamma Aminobutiric acid (GABA) inhibition way within the dorsal horn
As known, GABA is an essential regulator of spinal sensory neuron sensitivity, probably the most relevant inhibitory transmitter in CNS. This intracellular "GABA-glutamate-glutamine cycle" is maintained for normal physiological homeostasis. Hyperexcitable neurons and glial activation after spinal cord injury disrupts the balance of chloride ions, which reduces the hyperpolarizing (inhibitory) effect of GABA glutamate in the spinal dorsal horn. This vulnerable state exaggerates the effects of nociceptive stimuli and promotes the development of unpredictable and uncontrollable nociceptive sensitization. This uncontrollable noxious stimulation induces a form of maladaptive plasticity that enhances nociceptive sensitization and results in chronic neuropathic pain 31. Also, reducing the hyperpolarizing (inhibitory) effect of gamma-aminobutyric acid increases uncontrollable noxious stimulation that undermines the recovery of locomotor function, and increases behavioral signs of chronic pain, after a contusion injury. This adverse effect has been linked to a downregulation in brain-derived neurotrophic factor and an upregulation in the cytokine, tumor necrosis factor, enhancing tissue loss at the site of injury by increasing the extent of hemorrhage and apoptotic/pyroptotic cell death 32. Prevention of cell death of interneurons attenuates mechanical and thermal hyperalgesia, indicating that disinhibition contributes to neuropathic pain. Recent studies show that direct spinal implantation of forebrain GABAergic neuroprogenitor cells has been shown to alleviate neuropathic pain by integrating into the spinal cord pain circuit 16. Also, intrathecal lidocaine has demonstrated to block nociception-induced hemorrhage, cellular indices of cell death, and its adverse effect on behavioral recovery 32. Both papers confirm the role of GABA circuit in control of NP and the importance of inhibiting neural activity (nociceptive reactivity) following nerve trauma. Our evidence-based hypothesis is, alike to lidocaine, PRP could effectively control neuroinflammation and could contribute to the relief of NP, similar to lidocaine or even better, not only by blocking noxious inputs and avoiding the subsequent neural tissue damage but through its anti-inflammatory effect and its important role in nerve healing and regeneration 33,34, and considering the valuable effect of grow factors such as TGF-β1, alleviating early- and late-phase neuropathic pain symptoms, such as allodynia and hyperalgesia 17.
Central sensitization
Defined as abnormal increase of spontaneous and evoked activity of the CNS nociceptive neurons, central sensitization can appear at both the spinal and supraspinal levels and manifests as pain hypersensitivity, particularly dynamic tactile allodynia, and secondary punctate or pressure hyperalgesia. Therefore, central sensitisation might develop as a consequence of an ectopic activity in primary nociceptive afferent fibres but without structural damage within the CNS. This phenomenon can develop in most neuroinflammatory states, however, is self-limited and disappears with resolution of the primary cause, whereas it persists beyond the acute episode in many forms of NP. Contribution of central sensitization to NP has been well documented in many studies 35,36,37,38,39. Operationally, central sensitization appears as an amplification of neural signaling within the CNS that elicits pain hypersensitivity and contributes to inflammatory, neuropathic and dysfunctional pain disorders in patients 40. Therefore, the importance of preventing long-term changes that result from persistent injury to the nervous system, and eliminating the points where lesioned/ inflamed nerves are stretched or mechanically activated would be useful for lessening pain when this mechanism appears prevalent (ie, carpal tunnel syndrome, peripheral neuroma or trigeminal neuralgia) 41,42,43. Here as well, PRP could effectively control neuroinflammation and could contribute to the relief of NP.
Peripheral Mechanisms of Neuropathic Pain
Similar to spinal lesions which are associated with neuroinflammatory processes, lesión of a peripheral nerve is associated to a broad type of pathological changes involved in the mechanisms of NP 3.
Ectopic discharges in lesioned nerves and their corresponding dorsal root ganglia have been postulated to play a role in the mechanisms of NP. The ectopic expression of mechano-, thermo-, and chemotransducers along axons or in neuromas, can serve as aberrant points of action potential initiation. For example, mechanical pressure to which healthy nerves are generally insensitive can trigger ectopic discharges in underlying peripheral nerve lesions 44, developing tingling and paresthesia after gentle tapping (Tinel sign) or gentle mechanical compression (Phalen sign) as in carpal tunnel syndrome.
Ectopic expression of mechanoelectric transduction proteins on axonal lesion sites, have also been postulated to explain these phenomena. Neurotransmitter release by nociceptive terminals is triggered by Ca2+ entry and therefore depends on voltage-dependent channels. Confirmation of this voltaje-dependent hypothesis involved in NP origin is the antinociceptive effects of drugs, such as carbamazepine and its derivatives, phenytoin, lamotrigine, and lidocaine-like anesthetics, for which the main action is to stabilize Na+ channels by binding selectively to their inactive form, being efficacious in the treatment of certain forms of NP, and in certain types of NP, such as trigeminal neuralgia. These Na+ channel blockers are even a first-line treatment. Channel blocker properties also have been recently reported for other drugs, such as tramadol and fentanyl, used in the treatment of NP 45. However, side effects often limit their use.
Abnormal activity in axons undamaged by lesions distal to dorsal root ganglia lead to Wallerian degeneration, with the development of inflammatory phenomena, edema, and macrophage activation in the axonal segment disconnected from the soma.
Neuroimmune interactions resulting in enhanced and/or altered production of inflammatory signaling mo-lecules like cytokines and chemokines can cause sensitization of channels and result in firing of nociceptors.
Here our hypothesis is that PRP could produce sustained neuropathic pain-relief considering its neuroprotective and anti-inflammatory actions.
Conclusion to mechanisms contributing to NP is that, in response to injury, the nervous system undergoes changes in an attempt to re-establish synaptic transmission, not only at the injury site but also at all levels rostral to it, and NP is just one epiphenomenon resulting from these changes. However, a major obstacle in exploring mechanisms and treatments of neuropathic pain is that our conventional understanding of pain physiology and pharmacology has been built primarily on studies of nociceptive pain whereas persistent or neuropathic pain in many aspects differs from, and even is contrary to, nociceptive pain 46. Thus, interpreting pain as a simple pathway probably is not an enough explanation for the understanding of NP 32 and so, a better knowledge of its pathophysiological mechanisms Is a necessity when it comes to proposing an appropriate and definitive treatment.
Some concepts regarding peripheral NP diagnosis
In practice, the diagnosis of peripheral NP is a clinical one, relying on the patient's description of pain. Symptoms are a mosaic of hyperalgesia, allodynia, and sensory loss, described by the patient as prickling, deep aching, sharp, like an electric shock, and burning with hyperalgesia and frequently allodynia upon examination. However, no single symptom or sign is pathognomonic of NP 43, and there is no single diagnostic test for neuropathic pain. Ancillary studies are used to confirm or exclude underlying causes and suggest disease-specific treatments, such as for diabetes mellitus in patients with painful neuropathy or spinal disorders in patients with radiculopathy. Pain intensity is usually rated with any of several reliable and validated verbal, numerical, or visual analog scales. The quality of pain and not only the intensity is also desirable as abnormal sensations are frequent in patients with neuropathic pain 47. This can be assessed with measures of pain quality such as the McGill Pain Questionnaire used in the present study.
Current schemes treatments for NP and our cell-based proposal
Regarding current treatment of NP, systemically there is still controversy on the best management algorithm, and this varies depending on pain severity, underlying pathophysiology, and systemic comorbidities 2,4,5,6,7,8. General algorithm for neuropathic pain includes the use of alpha- 2- delta ligand antiepileptics (eg, gabapentin; pregabalin) as first-line agents, serotonin norepinephrine reuptake inhibitors (eg, duloxetine; venlafaxine) as second-line agents, and tricyclic antidepressants (eg, nortriptyline, amitriptyline) as third-line agents because of their side effects. Usually, multimodal therapies (antiepileptics and antidepressants) are needed because monotherapy most often provides only partial relief. In addition, depending on pain severity, opioids (eg, tramadol) can be used in selected patients, in conjunction with therapies above described. Topical agents (lidocaine and capsaicin) are also used even as first-line therapies or as parts of this multimodal therapy in specific conditions such as in the treatment of PHN. Short courses of corticosteroids or other anticonvulsants (such as topiramate, lamotrigine or, carbamazepine) are also included in many protocols. More aggressive measures (eg, nerve blocks, spinal cord stimulation) are used in patients who have failed conservative therapy or if there are specific indications, such as for neuropathic pain localized in the area of innervation of a specific nerve and related to neuropathy of that nerve (eg, sympathetically maintained arm pain treated with stellate ganglion block). But, as exposed at the beginning of this document, there is not an effective treatment for NP, despite all these proposed therapies.
Recently, biological therapy using PRP has been attracting more attention in the field of NP management 9,10,11,12. There are several techniques of preparation of PRP and different anatomic areas and different fields of application such as maxillofacial therapies, dentistry, wound healing, cardiothoracic surgery, neurosurgery, ophthalmology, cosmetics, and for tendon, ligament or muscle injuries. Likewise, PRP has been linked to anti-inflammatory and analgesic properties 48,49,50,51,52. However, the optimal dosage, frequency of administration, and appropriate stage of neuropathy for the use of PRP have not been investigated. Among all, the issue here is about the effect of PRP on neural tissue and the concern of its effectiveness in the management of peripheral neuropathies and the relief of NP. Studies arguing in favor of the use of PRP in neuropathies and/or NP, consider that PRP or stem cells can exert an anti-inflammatory effect through cytokine release, and consider the role of PRP or stem cells in nerve healing and regeneration, combating thus the pathological inflammation involved in neuropathic pain 53,54,55,56,57. This hypothesis has been tested in animal models with promising results, with a significant reduction in neuropathic pain symptoms. Likewise, good results have been obtained with PRP injected within the epidural space in patients with intervertebral disc disease (IDD) and central NP associated with promising results 58,59. Early relief of pain when PRP is injected into the epidural space may well be explained by its analgesic and anti-inflammatory effect on the dorsal root ganglion and not only the expected by its regenerative effect on the axial chondral tissue (intervertebral disc and facet joint), supporting the positive action of PRP in neuropathic pain (central NP in this case). This evidence based medicine (EBM) document is according to the conception of reasonable use of modern, best evidences in making decisions about treatment of individual patients 60,61,62,63,64,65,66,67,68, trying to give our patients the best possible solution to this pressing clinical problem.
CONCLUSIONS
In response to injury, the nervous system undergoes changes in an attempt to re-establish synaptic transmission and these changes entail functional modifications not only at the site of injury but also at all levels rostral to it, and NP is just probably one epiphenomenon resulting from these changes. The effectiveness of treatment for patients with NP should consider, therefore, a better understanding of its pathophysiological mechanisms.
Currently, there are no drugs that can treat neuropathic pain in a complete and definitive way. Research on the pathophysiologic mechanisms of neuropathic pain predicts the near future application of a more effective and specific mechanism-based treatment approach. Thus, an entirely different strategy in which pain is differentiated on the basis of the underlying mechanisms is the novel proposal.
Considering the factors associated with current pharmacological ´consensus-based' schemes, like the potential for adverse side effects, drug interactions, and even risks of misuse and abuse when opioids are prescribed, a direct comparison of the diverse pharmacological regimes with PRP therapy makes it difficult to contrast, if based on efficacy, safety, and tolerability. PRP could offer distinct advantages for the efficacious treatment of chronic NP. This cell-based method of treatment reduces pain intensity, is a safe and well tolerated option and, as it is derived from the patient own blood, the risk of adverse or allergic reactions are nule.
Critical to exploring the role of PRP in treating neuropathic pain and to assessing whether PRP is effective, further clinical studies should be performed in the future, in a randomized controlled trial, as PRP mechanism of action in relieving pain is not yet clearly elucidated. Likewise, various questions are still to be resolved with PRP therapies, such as there could be a better result in pain relief by increasing the number of injections.
Similar to other reports in which this novel cell-based therapy with PRP has been used in the management of NP, this study is just a small case series. However, the positive and promising results with PRP in treating peripheral neuropathic pain in the present study encourage us for further clinical investigations.