My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Sociedad Española del Dolor
Print version ISSN 1134-8046
Rev. Soc. Esp. Dolor vol.26 n.6 Madrid Nov./Dec. 2019 Epub Mar 30, 2020
https://dx.doi.org/10.20986/resed.2019.3715/2018
REVIEW
Neurolytic procedures for pancreatic cancer pain: a systematic review and a proposal for an algorithm treatment
1Centro Integral de Dolor Oncológico CIDO. HM Hospitales.
Pancreatic cancer provokes pain in more than 80 % of patients, resulting in a management of pain that is often unsatisfactory due to the limited treatment options and the significant variation in clinical practice, emphasizing the need for a multidisciplinary approach. This article has been redacted to review the literature and summarize the actual evidence of neurolytic procedures to treat pain caused by pancreatic cancer. The sources of these of articles have been obtained by using PubMed and Medline, restricting the search to randomized control comparative studies, systematic reviews, prospective and retrospective studies, and case series presentations. This article shows the actual evidence of the different approaches for the celiac plexus and splanchnic nerve neurolysis, regarding its efficacy, risks, adverse effects, and limitations. The final objective has been to propose an interventional algorithm that might help to improve pain management in patients suffering from pancreatic cancer.
Key words: Neurolysis; pancreatic cancer; celiac plexus; splanchnic nerves; endoscopic ultrasound; cancer pain
INTRODUCTION
Pancreatic cancer is one of the solid tumors with the worst prognosis that exists; ductal adenocarcinoma affects more than 90% of pancreatic cancer cases. The 5-year survival is only 5-10% and more than half of the patients do not survive after the first year 1. This poor prognosis occurs because the symptoms usually appear late, causing that only 10-20% of pancreatic adenocarcinomas are resectable at diagnosis. More than 80% of patients will suffer abdominal and posterolumbar pain throughout their illness, 50-70% referring severe pain 2, largely because this type of cancer has a range of perineural infiltration of 80-100% 3. It is therefore essential to control the pain of these patients, since it often presents as a challenge for doctors due to its high complexity and its poor evolution.
The presence of any of the following situations implies a contraindication for intent-to-treat surgery in pancreatic adenocarcinoma 4: Distant metastasis; absence of the normal fat plane between the tumor and the celiac trunk; boxing of the superior mesenteric artery above 180° of its circumference; invasion of the vena cava and/or aorta, unresectable involvement of the mesenteric-portal axis; and lymph node involvement outside the standard resection field. When any of these situations occur and the pain becomes severe and refractory to medical treatment according to the guidelines of the World Health Organization (WHO) 5, we can use interventional procedures that have proven to be highly effective and safety profile, with a low incidence of serious complications. The procedures range from neurolysis of the celiac plexus and splanchnic nerves, with their various approaches and imaging techniques, to spinal infusion and neurostimulation. This review tries to clarify the indications, effectiveness, advantages and disadvantages of neurolytic techniques aimed at relieving visceral pain associated with pancreatic cancer.
ANATOMICAL TARGETS
The visceral pain of the upper abdomen is collected by a special set of afferent nerve fibers clustered in the celiac plexus and splanchnic nerves 6:
The celiac plexus is located retroperitoneally on the anterior face of the aorta, at the level of T12 and L1 vertebrae, in front of the diaphragmatic crura. This plexus is formed by two or more ganglia arranged around the celiac trunk and the superior mesenteric artery, receiving sympathetic fibers from the splanchnic nerves and parasympathetic fibers from the vagus nerve. The ganglia receive the afferents of the sympathetic fibers of the digestive tract that go from the distal third of the esophagus to the splenic angle of the colon, including sympathetic fibers of the liver, pancreas, gallbladder, spleen, kidneys, proximal ureter and adrenal glands, as well as the blood vessels that form the celiac plexus.
The splanchnic nerves are three nerves that are born from the thoracic sympathetic chain and are arranged laterally in the spine: 1) The greater splanchnic nerve is formed by the sympathetic preganglionic fibers from T5 to T9, 2) the minor splanchnic is formed by the fibers from T10 and T11, and 3) the inferior splanchnic, by the fibers coming from T11 and T12. All of them converge on the lateral face of the T11 and T12 vertebrae - ideal place for the performance of a neurolysis - and run parallel until they cross the diaphragm and join the celiac plexus.
NEUROLYTIC INTERVENTIONAL PROCEDURES
To prescribe neurolysis in a malignant tumor process, patient selection is crucial. The most important variables to consider are: 1) pain severity, 2) persistent pain despite medical treatment, with chemotherapy or radiotherapy, 3) mainly visceral pain, 4) pain that cannot be treated by other less invasive procedures, and 5) a reduced life expectancy of patients.
There are factors influencing the outcome of the procedure, such as: 1) The image used (fluoroscopy, computerized tomography (CT scan), ultrasound or endoscopic ultrasound); 2) the volume and concentration of the neurolytic agent, since the increase in both can improve the effectiveness of the blockage, but also involves a larger risk of complications 7; 3) the location of the tumor in the viscera, being neurolysis more successful if the tumor is in the head of the pancreas (92%) than if it is in the pancreas body or tail (29%) 8; 4) the extension of the tumor mass, since if it infiltrates the celiac plexus 9, somatic areas (peritoneum, diaphragm) or produces neuropathic pain, the success rate can be significantly reduced; and 5) the previous diagnostic block as a positive predictive factor, but questionable if it is negative in patients with terminal cancer 10.
Among the general contraindications for the neurolysis of the celiac plexus and splanchnic nerves are: coagulation disorders (INR> 1.5, thrombocytopenia <50000); concomitant treatment with antiplatelet agents and/or anticoagulants 11); the presence of intestinal obstruction, due to the sympatholytic effect of the blockage; the inability of the patient to remain in supine or prone position depending on the approach; and the tumor invasion of the celiac trunk when our target is the celiac plexus.
Considering that our targets are the celiac plexus and/or the splanchnic nerves, we can distinguish interventional procedures according to the approach in the space, the imaging technique used, and the type of percutaneous approach:
According to the approach in the space
- Posterior: most commonly used classical approach, performed by fluoroscopy or CT scan, with the patient in prone position.
- Anterior: it can be performed through endoscopic ultrasound, percutaneous needle guided by ultrasound or CT scan, or intraoperatively by laparotomy. The anterior percutaneous approach is performed in supine position and requires antibiotic prophylaxis due to the risk of perforation of intestinal viscera.
According to the imaging technique used
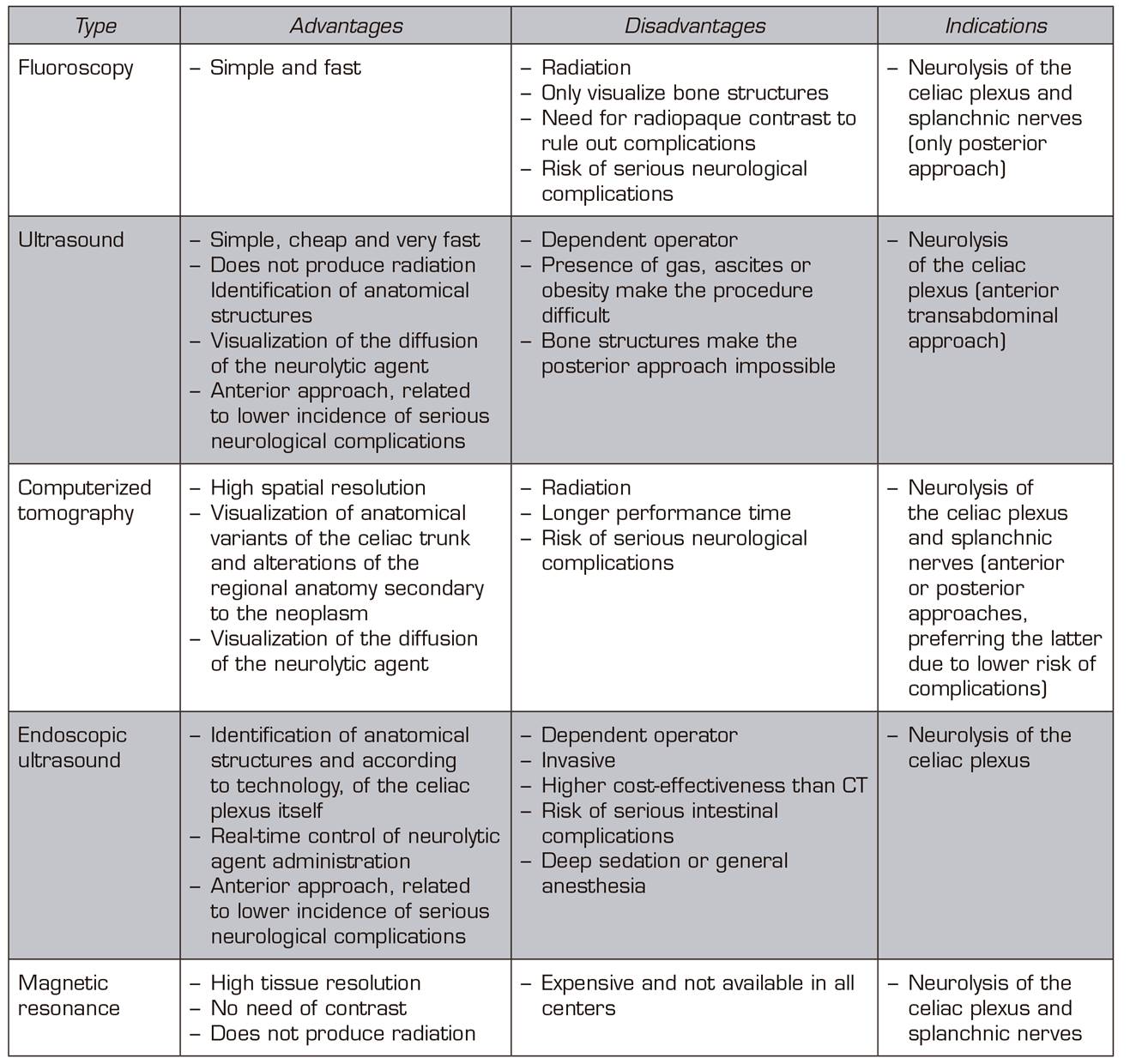
Each imaging technique has advantages, disadvantages and indications, which are summarized in Table 1.
According to the percutaneous approach
Diaphragmatic crura will determine anatomically whether the blockade performed represents a true celiac plexus block or if it is a splanchnic nerves block (Figure 1). If the tip of the needle is placed after the crura, the splanchnic nerves will be blocked. The needles arranged at the level of the vertebral body of T11 will always be behind the crura. Below this level, the crura becomes posterior and it is inserted into the vertebral bodies of T12 and L1. At this level, the needles can be placed both anterior and posterior to crura. The classic approach of Kappis 12 of needle positioning at the anterior edge of the vertebral body usually results in a retrocrural block, demonstrated in subsequent studies on dead bodies with CT scan 13. To achieve a true block anterior to crura, the needle must be moved further so that it is anterior to the abdominal aorta.

Fig. 1. Diaphragmatic crura and its anatomical arrangement as a boundary between retro and transcrural approaches.
Once this issue is clarified, we can classify percutaneous approaches as follows (Figure 2):
Transcrural approach: the most commonly used for the blockage of the celiac plexus (Figure 3). The patient is placed in prone position, identifying the vertebral body L1 and advancing a needle on each side, approximately 7.5 cm from the midline, until it crosses the diaphragmatic crura and blocks the plexus.
Retrocrural approach: an approach that has been classically described for both the celiac plexus block (at the L1 level) and for the splanchnic nerves. In the pure retrocrural approach, the patient is placed in prone position and the vertebral bodies of T11 and T12 are located, advancing the needles to the anterior third of these bodies and thus blocking the splanchnic nerves.
Transaortic approach: described by Ischia et al. 14, it is a unilateral approach with the patient in prone position, accessing from the left side of the vertebral body of L1 until crossing the aorta and leaving the tip of the needle anterior to it. Blood output will be appreciated at the moment in which we cross the artery, with a posterior cessation as soon as we are anterior to it. It has been found that the risk of occult bleeding is low since in this area the aorta is reinforced by diaphragmatic crura and prevertebral fascia. However, publications are scarce and studies of larger scientific evidence are needed. Specific contraindications for this approach are the presence of abdominal aortic aneurysm, mural thrombosis and calcifications.
Transdiscal approach: it is performed under CT monitoring through the intervertebral disc T12-L1 until reaching the splanchnic nerves. Although the literature is scarce, this approach theoretically reduces the risk of complications such as paraplegia, pneumothorax and liver or kidney perforation, since the needle is inserted closer to the midline, being very useful in patients with anatomical abnormalities around of the celiac plexus or in patients with organomegalies 15,16.
Abdominal approach: normally used under ultrasound vision by anterior route.
CLASSICAL PERCUTANEOUSCELIAC PLEXUS NEUROLYSIS
Percutaneous celiac plexus neurolysis consists in the destruction of the fibers composing the celiac plexus by injecting a neurolytic agent, the use of alcohol is preferred due to its lower affinity for blood vessels compared to phenol. The most commonly used classical approach is the bilateral transcrural posterior, guided by fluoroscopy or CT.
In recent years, several randomized controlled trials have emerged that have been analyzed in a systematic review conducted by Mercadante et al. 17, demonstrating a larger analgesic effectiveness in a significant, although minimal, in the groups in which the celiac plexus neurolysis was performed compared to the groups treated with systemic opioids. In addition, they demonstrated a reduction in opioid consumption in most studies 1819202122232425262728-29 and an improvement in the quality of life in some of them 20,27,29. Complications were rare for all approaches, although it was not a primary variable in any study. In spite of these results, the quality of all the studies, with the exception of two 24,28, was poor due to important limitations, such as sample size, allocation concealment or time to evaluate the effectiveness of the blockage.
Complications derived from celiac plexus neurolysis have been published, being uncommon in most cases 30. It is necessary to know how to differentiate the expected adverse effects of chemical sympathectomy from the complications derived from the technique. A total of 20-42% of patients have hypotension due to vasodilation secondary to neuolysis. Diarrhea has an incidence of 10-25%, resolving in the first 48-72 hours. The presence of low back pain has an incidence that ranges from 5% to 60% depending on the series. Omalgia is also described in 1% of cases, due to diaphragmatic irritation. Regarding the percutaneous approach, complications of the technique are rare (2%), being neurological deficit (weakness and extra sensations), hemorrhagic gastritis, duodenitis, pneumothorax, hematuria and death described (3.1) %). Paraplegia is published with posterior approaches (0.15%), and may be due to direct needle injury of the spinal cord or due to spinal infarction secondary to arterial spasm.
PERCUTANEOUS ULTRASOUND-GUIDED CELIAC PLEXUS NEUROLYSIS
In recent years, ultrasound has made head against fluoroscopy and CT in interventional pain management. The first describing the technique on the celiac plexus was Bhatnagar 31, and it could be performed percutaneously at the patient's bedside and supine, which gives greater comfort for both the patient and the doctor. However, there are still no randomized controlled trials comparing abdominal ultrasound neurolysis of the celiac plexus versus opioid systemic treatment. The technique is performed using a convex transducer (2-5 MHz), initially ºº splenic arteries, and more distally in the superior mesenteric artery. Then we rotate the transducer in the longitudinal plane, visualizing in the same image the bifurcation of the aorta in the celiac trunk and in the superior mesenteric artery with the celiac plexus surrounding these structures. The approach is performed in plane, transabdominal, through the liver or stomach, as long as there are no large vessels interfering with the needle path (Figure 4).
No complications have been reported with the percutaneous ultrasound approach; however, the literature in this regard is insufficient to draw conclusions 32.
DIFFERENCES BETWEEN PERCUTANEOUS APPROACHES FOR THE CELIAC PLEXUS NEUROLYSIS
There are no differences in terms of pain control in the short and long term. Ischia et al. 33 conducted a comparative and randomized study, no differences in the level of analgesia were found when they compared between three posterior approaches (retrocrural, transaortic and chemical sclechnicectomy), obtaining significant pain relief of 70-80% immediately and 60- 75% until death. However, the groups were small and opioid consumption was not evaluated. The remaining studies are of poor quality: Tewari et al. 34 showed superiority with the retrocrural block of the celiac plexus over the transaortic block, although the retrocrural block was probably acting on the splanchnic nerves. Marcy et al. 35 showed a control of pain in 27 out of 34 patients with a similar success with the abdominal ultrasound approach compared with CT-guided (93% versus 100%), preferring the path through the liver, with only minor complications. In another randomized controlled study comparing the same approaches 36, the success of the technique and the quality of life of the patients was similar, but the CT-guided approach required fewer attempts and repetitions of the blockage. Therefore, there is not enough scientific evidence at the moment to choose one percutaneous technique instead of another.
ENDOSCOPIC ULTRASOUND NEUROLYSIS OF THE CELIAC PLEXUS
Endoscopic ultrasound offers the advantage of larger visualization of the celiac plexus at a short proximity, allowing larger precision and safety in the administration of the neurolytic agent and avoiding injection into vascular structures through the use of Doppler 37 (Figure 5). However, the studies supporting endoscopic ultrasound neurolysis, considered similarly to percutaneous technique as a rescue therapy, are limited to retrospective uncontrolled studies (38). Pauli et al. published a meta-analysis that concludes that this technique achieves an 80% pain reduction in patients with pancreatic cancer 39. Some studies show a slight decrease in opioid consumption, but without strong scientific evidence.

Fig. 5. Endoscopic ultrasound imagen of celiac plexus neurolysis. Ao: abdominal aorta, TC: celiac trunk, PC: celiac plexus, AMS: superior mesenteric artery.
To date, no studies have demonstrated an increase in survival after celiac plexus neurolysis using the endoscopic ultrasound technique. There is a retrospective case-control study, which concludes that neurolysis is independent from the survival of pancreatic cancer patients 40, but studies providing more evidence are needed.
Regarding adverse effects, they are reported in a limited series of retrospective publications and case series, being hypotension (11%), diarrhea (18%), and transient abdominal pain (1.5 to 8%) described 37. Theoretically, this technique is safer, with its anterior approach through the gastric wall and direct passage of the needle to the plexus visualizing the vessels, without having to cross the retrocrural space 41. Even so, fatal complications have also been published with this technique. Gimeno-García et al. 42 described the first complication of thrombosis and vasospasm of the celiac artery that produced a multiorganic ischemia and the death of the patient. Since then, this complication has been published in 2 other studies, which also ended in death 43,44. Other adverse effects described are retroperitoneal bleeding and 2 cases of paraplegia.
Among the contraindications related to this technique the following are found: the presence of gastric or esophageal varix - since they increase the risk of bleeding-, an unstructured anatomy, direct tumor invasion of the plexus, and congenital malformations of the celiac trunk or of the superior mesenteric artery.
PERCUTANEOUS OR ENDOSCOPIC ULTRASOUND APPROACH OF THE CELIAC PLEXUS?
There are no studies that directly compare the two approaches in pancreatic cancer patients. There are only two randomized controlled trials comparing the percutaneous approach - one with CT and the other with fluoroscopy - versus the endoscopic ultrasound approach, but in patients with chronic pancreatitis 45,46, demonstrating larger effectiveness in pain control for the endoscopic ultrasound procedure, but only in the first 4 weeks, without differences at 8 or 12 weeks, and without differences regarding adverse effects, consistently with the systematic review of Nobre Moura et al. 47. In addition, these studies did not use neurolytic agents but local anesthetics and corticosteroids, since they were patients with benign pathology.
SPLANCHNIC NERVES NEUROLYSIS
The splanchnic nerves are located in the anterolateral face of the vertebral bodies from T9 to T11, above the insertion of the diaphragmatic cruras, constituting an important barrier for situations that may increase the failure of a celiac plexus neurolysis (variables or anatomical changes, fibrosis, adhesions or tumor infiltration) 16. In these cases, splanchnic nerve neurolysis can be very useful, and it can be performed surgically or percutaneously.
Surgical technique
The surgical technique is performed through thoracoscopy with the patient in prone position. The advantages of this technique include the possibility of performing the neurolysis bilaterally by insufflation of CO2 and insertion of the two trocars at the same time, and the high precision in the identification of the splanchnic nerves, being able to target T5 to T12. It can be performed without selective pulmonary ventilation, although a high percentage of surgeons prefer it because it facilitates the technique and reduces surgical time. The disadvantages to highlight are the presence of pleural adhesions that difficult the procedure and the risks associated with general anesthesia.
Literature is limited to prospective studies and case series 48. In a study comparing between patients with pancreatic cancer and patients with chronic pancreatitis, Bhutiani 49 showed a larger analgesic effectiveness with lower opioid consumption and hospitalization in the cancer group than in the chronic pancreatitis group.
Percutaneous technique
The percutaneous technique is performed using a retrocrural posterior approach, usually guided by fluoroscopy (Figure 6). The lesions can be performed by conventional radiofrequency or using neurolytic agents as in the celiac plexus.

Fig. 6. Fluoroscopy-guided posterior percutaneous neurolysis of splanchnic nerves, at T11 and T12 levels.
Conventional or thermal radiofrequency of the splanchnic nerves
It consists of the production of a high-frequency electric current at the tip of the needle, generating heat around it and producing tissue destruction if the 45-50 °C and 20 seconds of temperature stabilization are exceeded, influenced also by other factors, such as the gauge of the needle and the length of the active tip 50. For splanchnic nerves, usually needles of 18 to 20G are used, with a minimum active tip of 10 millimeters, establishing lesions of 80 °C and 90 seconds per needle 51.
Conventional radiofrequency of the splanchnic nerves is widely documented in the literature with studies that have demonstrated its effectiveness in pain associated with chronic pancreatitis, but not in pancreatic cancer. This is due to the more predictable radioanatomy of the splanchnic nerves and the lower rate of severe complications, since the technique allows a previous sensory and motor stimulation to avoid injury to other nerves, and does not necessarily require neurolytic agents that can disseminate and affect other structures 51,52. Regarding pancreatic cancer, a retrospective study conducted in 56 patients showed a significant decrease in the visual analog scale (VAS) and in opioid consumption, with an improvement in the quality of life of patients up to six months, being the effect more extended than with the use of neurolytic agents 53. However, randomized controlled studies are needed to achieve relevant conclusions.
Chemical neurolysis of the splanchnic nerves
Recently, the administration of neurolytic agents in the splanchnic nerves has again gained interest due to its lower anatomic variability in relation to surrounding structures and its greater percutaneous accessibility 54,55,56. However, so far there is no superior evidence of chemical neurolysis compared to conventional radiofrequency in patients with pancreatic cancer.
Amr et al. 57 conducted a comparative study in patients with pain associated with pancreatic cancer and other neoplasias of the upper abdomen, comparing the chemical neurolysis of splanchnic nerves with conventional radiofrequency. The decrease in VAS was more important in the group treated with radiofrequency, with a decrease in opioid consumption and with an improvement in quality of life similar in both groups, without the presence of serious complications. This is a small study and more studies offering more scientific evidence are needed.
Complications in the approach to splanchnic nerves are uncommon. Similarly to neurolytic blocks, radiofrequency can also cause neuritis that usually disappears within a few weeks. Hypotension and diarrhea are self-limited and derive from sympatholysis, although less frequently than with the celiac plexus block. As in all thoracic procedures, it is necessary to be alert of the possible pneumothorax, recommending a subsequent radiographic control. The sensation of dyspnea that patients sometimes present may be due to anesthetic blockage of the phrenic nerve, resulting in an elevation of the hemidiaphragm. Other rare complications reported in the literature are thoracic duct lesion, suspicious when yellowish and cloudy fluid is aspirated through the needle, or intradiscal and intravascular injection, which should always be previously verified with radiopaque contrast. Paresthesia may appear if there is needle contact with thoracic dorsal roots 58. The use of radiofrequency in the splanchnic nerves should avoid the risk of paraplegia 51, but the results have been poorly studied.
Among the specific contraindications for the destruction of splanchnic nerves we found the following: the existence of an abdominal aortic aneurysm, respiratory failure, unilateral pneumothorax and the presence of pleural adhesions.
NEUROLYSIS OF THE CELIAC PLEXUS OR NEUROLYSIS OF THE SPLAnchnic NERVES?
The vast majority of studies published to date on the interventional management of pain associated with pancreatic cancer have focused on the chemical neurolysis of the celiac plexus, including the two with the highest scientific quality 24,28, demonstrating its superiority over pharmacological therapy. The first comparative study between the celiac plexus neurolysis - through a transaortic approach - and splanchnic nerve neurolysis in pancreatic cancer patients was conducted by Ozyalcin et al. 59, showing statistically significant differences in favor of splanchnic neurolysis, and surprisingly a lower survival rate for the group treated on the celiac plexus. Then, Shwita 56 published another similar comparative study, with a larger number of patients and 2 years of follow-up, although it included different oncological pathologies of the upper abdomen, showing a similar analgesic effectiveness for both groups in the first weeks, with better opioid management of the pain and better quality of life in the splanchnic neurolysis group from the fourth month. The decrease in opioid consumption was similar in both groups.
Plancarte et al. (54) described the chemical neurolysis of splanchnic nerves as an alternative to celiac plexus neurolysis when anatomic changes are present in the area. This was the first paper published in PubMed explaining the transdiscal approach to these nerves. Their observational study included 109 patients with malignant upper abdominal pain and showed a decrease in VAS score and opioid use, with sustained clinical improvement until one year of follow-up or patient death, and without technique-related serious complications. Subsequently, Ahmed et al. 56 performed a small retrospective study that included patients with different abdominal neoplasms (biliary, gastric, pancreatic), and obtained results similar to those of Plancarte et al. 54.
Marra et al. 60 presented a series of 150 cases undergoing chemical neurolysis of the celiac plexus, splanchnic nerves, or both, through an anterior approach guided by CT, obtaining better results in patients undergoing splanchnic nerve block and combined block compared to those undergoing only the celiac plexus block.
Regarding thoracoscopic procedure of the splanchnic nerves, some studies compare the effectiveness of videothoracoscopy against the neurolysis of the celiac plexus. Stefaniak et al. 27 investigated the severity of pain, quality of life and consumption of opioids in 35 patients treated with celiac plexus neurolysis and 24 patients treated with unilateral thoracoscopic splanchnicectomy, concluding that both procedures provided similar effectiveness, preferring celiac plexus block because it is less invasive and it improved the quality of life of patients more than the videothoracoscopy. Furthermore, Johnson et al. 61 compared the effectiveness of the bilateral neurolysis of the celiac plexus, thoracoscopic splanchnicectomy and medical opioid therapy in 65 patients with upper abdominal cancer pain, with a two-month follow-up. The conclusions of this study, in contrast with the other studies, is that the two interventionist approaches did not obtain sufficient decrease in pain or opioid consumption compared to the group treated only with opioids.
NEUROLISIS WITH ALCOHOL OR WITH PHENOL?
Alcohol and phenol are the two agents used for the chemical neurolysis of the sympathetic chain 62. Ethyl alcohol is a colorless and hypobaric solution compared to plasma, diluting it to 50-70% to obtain a sympatholytic effect. Phenol is not marketed in Spain and it must be previously prepared in the hospital pharmacy. Phenol is unstable at room temperature and its half-life is 1 year when it is kept cold and away from light. It is usually used at concentrations of 6-10% for sympathetic blockages, since below 2% it only produces an anesthetic effect and above 20% it can destroy somatic nerves. The chemical properties and differences between both agents are summarized in Table 2.
Studies comparing alcohol with phenol in sympathetic blockages are scarce. Koyyalagunta et al. 63 published a retrospective study of 93 abdominal cancer patients undergoing chemical neurolysis of the splanchnic nerves, assessing the effectiveness, duration of blockage and adverse effects of both agents. They found no differences in pain relief or the incidence of complications. Tumor infiltration of the celiac trunk and previous radiotherapy did not interfere with the effectiveness of the procedure, which is why the researchers chose the splanchnic nerves as the target.
There is a large literature variety about what type of agent to use and how much volume to administer, basically depending on the target chosen, the percutaneous approach, and the imaging technique we use. For blockages performed transcrurally, about 15-20 ml of neurolytic agent is recommended on each side, with alcohol being preferred because of its lower affinity for blood vessels. For the abdominal ultrasound approach, 8-10 ml of alcohol is sufficient. For the neurolysis of the splanchnic nerves, 6-8 ml of phenol is recommended, due to its lower affinity for the somatic nerves. If we use the endoscopic ultrasound technique, the volume is also low. LeBlanc et al. 64 found no differences between the endoscopic ultrasound administration of 20 ml versus 10 ml of alcohol.
NEED A PREVIOUS DIAGNOSTIC BLOCK?
The usual procedure for any percutaneous interventional technique producing tissue destruction is to perform a diagnostic block. However, regarding incurable oncological diseases, this algorithm can be discussed. Yuen et al. 10 published a retrospective study comparing patients with abdominal cancer undergoing celiac plexus neurolysis after a positive diagnostic block compared to patients in whom neurolysis was performed directly. They concluded that a positive response to the diagnostic block was positively correlated with the neurolytic block, but the diagnostic block was a poor predictor when the response was negative. Therefore, its clinical role may be questionable in patients with terminal cancer, although more comparative studies are required to support this conclusion.
Moreover, no diagnostic block is performed prior to the endoscopic ultrasound neurolysis, probably because it is a more invasive procedure, but it is favored because it obtains a better visualization of the structures and of the neurolytic agent.
UNILATERAL OR BILATERAL NEUROLYSIS?
Unilateral neurolysis consists of a single injection at the base of the celiac trunk, but involves the risk of inadequate exposure of the plexus, considering that it is located slightly to the left of the aorta; and if we use endoscopic ultrasound, in most cases it is between the celiac trunk and the left adrenal gland. Bilateral neurolysis consists in the administration of the neurolytic agent on both sides of the celiac plexus, either by a posterior percutaneous approach or by rotating the ultrasound endoscope on each side of the celiac trunk 37. P Although the bilateral approach could be associated with a higher risk of complications, it has been shown to have better results in the reduction of pain in patients even in the duration of the neurolytic block, with an incidence of complications similar to that of the unilateral approach, for both classical percutaneous technique and endoscopic ultrasound 64,65. In a recent meta-analysis 66, no differences between the unilateral or bilateral endoscopic ultrasound technique are found, but a decrease in the need for postoperative analgesics in the bilateral neurolysis group is shown. In any case, it must be considered that most meta-analyzes are performed with the bilateral approach, for both the classical percutaneous procedure and the endoscopic ultrasound 67.
Ultrasound-guided percutaneous neurolysis has also been published in a randomized study 68, comparing the unilateral paramedial versus bilateral approach in patients with abdominal oncological pathology, without finding differences in pain relief and its duration of up to 3 months, but with larger discomfort of the patient using the bilateral approach. The limitations of this study were that it was not a double blind - the patient was awake during the technique - and, to date, there are no studies demonstrating the superiority of abdominal ultrasound compared to other imaging approaches and techniques.
CENTRAL NEUROLYSIS
Central neurolysis consists of the direct injection of the neurolytic agent in the celiac plexus ganglia, being possible only by endoscopic ultrasound, thanks to the technological development of the last years. There are prospective studies showing the ability to detect ganglia between 81 and 89% of cases 69,70. There is a randomized controlled trial comparing the unilateral endoscopic ultrasound neurolysis with the central neurolysis, demonstrating larger pain relief in the central neurolysis group (75% versus 45.5%), but there are no trials comparing the bilateral approach with the central approach 71. Central neurolysis of the celiac plexus is dependent on the technology of the instrumental equipment and it is also presumable that when the neurolytic agent is injected into the ganglion, the drug can diffuse beyond its target and destroy other non-visible ganglia. Therefore, bilateral neurolysis remains the main approach in patients with pancreatic cancer.
ENDOSCOPIC ULTRASOUND RADIOFREQUENCY ABLATION OF THE CELIAC PLEXUS
Recently, radiofrequency ablation of the celiac ganglia by endoscopic ultrasound has emerged as another alternative to alleviate pancreatic cancer pain. There is a randomized controlled study comparing the endoscopic ultrasound-guided celiac ganglion radiofrequency ablation radiofrequency with the celiac plexus neurolysis 72, with larger pain management and quality of life of patients treated with radiofrequency, although the sample size was small and the follow-up was only 4 weeks, so more comparative studies on this subject are needed.
EARLY OR LATE NEUROLYSIS?
Celiac plexus neurolysis is not recommended before surgery due to the risk of fibrosis and other complications. For unresectable tumors, the potential advantage of an early neurolysis is to prevent and minimize the progression of pain and tolerance to opioids. However, it seems that the analgesic effect usually disappears after 8 weeks and in most patients the pain recurs at 3 months 73. After this period, the celiac plexus neurolysis could be repeated without increasing the risk of complications, but with an effectiveness rate that can drop to 30%, as shown by the study of McGreevy et al. 74.
Some studies question the algorithm proposed by the World Health Organization (WHO) on the use of invasive methods only when the third step fails. In a comparative study, Amyr et al. 75 showed a larger pain management at 2 months, associated with a better quality of life and lower consumption of opioids, in a group of patients with pancreatic cancer undergoing neurolysis after treatment with systemic opioids compared to another group of patients undergoing neurolysis before starting with opioids. Interestingly, the same researchers concluded in a subsequent study conducted in 109 patients with abdominal and pelvic cancer 76, a statistically significant increase in responders to sympathetic neurolysis when performed before the second step of the WHO compared to another group that followed the normal algorithm, with a larger decrease in adverse effects and opioid consumption of up to 12 months in the first group.
Regarding the doses of opioids, De Oliveira et al. 29 found no significant differences in neurolytic blockade of patients with high doses of morphine (> 90 mg/day of oral morphine) compared to those with low doses (<90 mg/day of oral morphine). There is a prospective study on the early percutaneous ultrasound-guided approach for pain management in gastrointestinal and pelvic malignancies 31 that included a total of 44 patients undergoing celiac plexus neurolysis, superior hypogastric and ganglion impar: To be considered an "early neurolysis ", the inclusion criteria of the patients should be having a VAS > 7 or being under treatment with oral morphine > 30 mg/day in the month prior to the blockage. VAS was significantly reduced, with only 11.4% of patients needing oral morphine after the procedure, although the follow-up was only for two months.
Regarding the endoscopic ultrasound technique, there is published a randomized controlled trial that compares early endoscopic ultrasound neurolysis with systemic analgesia, being more significant pain relief in the neurolysis group, with a follow-up of up to 3 months, but with a less significant difference in opioid consumption between both groups, influenced also by if patients received chemotherapy or radiotherapy 28.
DISCUSSION
Unlike other abdominal-pelvic cancer pain, visceral pain associated with pancreatic cancer has the advantage of having well defined anatomical targets in which to act on the sympathetic axis, with studies demonstrating pain relief in more than 70-90% of patients, showing a slight superiority compared to systemic drug treatment and a lower incidence of adverse effects. However, there are still controversies about which approach and which imaging technique are more suitable for these patients. The ideal intervention procedure would be that which complies with the following characteristics: 1) be minimally invasive, 2) be associated with a minimal risk of serious complications, 3) achieve significant pain relief, 4) improve the quality of life of patients, 5) extend the survival of patients and 6) produce a decrease in opioid consumption.
To date, the studies with larger scientific evidence have been conducted on the celiac plexus (level of evidence 2 A+ with grade of recommendation II B) 77,78, most of them using the posterior percutaneous approach although almost all of them had important limitations. According to the results of the study conducted by Mercadante et al. 17, it can be concluded that the celiac plexus neurolysis has a higher effectiveness than systemic opioid treatment, being minimal, but with much less frequent adverse effects, something that is important for patients. Another important fact found in the same review is that no significant differences were found in VAS after 2 months of follow-up, although there were only 2 studies assessing pain up to 3 months after neurolysis 24,26. The decrease in opioid consumption was significant in all studies but only up to 2 months of follow-up, and only one study continued finding this decrease 3 months after neurolysis 26. Therefore, these results reflect that the effect of neurolytic blockade can last between 2 and 3 months 73, something important to consider when deciding on the appropriate time to perform the technique.
Neurolytic blockage of the celiac plexus could theoretically extend the survival of pancreatic cancer patients. Ductal adenocarcinoma has neurotrophic characteristics, and as tumor growth follows the path of the nerves, the destruction of the nerves could be advantageous. However, there is only one randomized controlled trial showing a larger survival in patients receiving celiac plexus neurolysis compared to a group receiving saline 19, although this study was conducted in patients undergoing exploratory laparotomy, with in situ diagnosis of unresectable tumor and direct injection of the chemical agent by the surgeon. Staats et al. 22 published a study showing an increase in survival after celiac plexus neurolysis, but this result could not be reproduced in subsequent retrospective studies 79, so there is not enough evidence supporting that the neurolytic blockade affects disease progression.
Furthermore, the evolution of the work teams together with the specialization of the medical operators have motivated the development of new interventional techniques, such as the endoscopic ultrasound neurolysis (level of evidence B with grade II A) 79 and the ultrasound-guided percutaneous neurolysis of the celiac plexus. These procedures improve the visibility of the anatomy around the plexus and the precision when injecting the neurolytic agent, and can reduce related complications. Ultrasound-guided percutaneous neurolysis can also be conducted at the patient's bedside, reducing surgical time and stress of the patient. However, no studies with sufficient scientific quality supporting these procedures over the previous ones are available to date. No sufficient evidence supporting neurolysis of the splanchnic nerves (level of evidence 2 B+ with grade of recommendation II B+) 78 compared to the celiac plexus, although the few published studies suggest similar analgesic effectiveness, with a better quality of life of patients and a potential more extended pain relief if we use conventional radiofrequency, waiting for more comparative studies to be published to obtain more accurate conclusions.
There are still controversies about the appropriate time to perform the neurolysis. Some studies suggest an early intervention 2021-22,29,33, while others, such as De Oliveria et al. 29, showed no significant differences between patients treated with high doses of opioids compared to others treated with low doses. It should be clarified that the level of opioid consumption should not reflect in any case the stage of the disease. Amr and Makharita 75 obtained better results with celiac plexus neurolysis if pain was controlled priorly with systemic medication. To decide when we can perform the neurolysis, the main factors to consider are the duration of the effectiveness of the blockage, the tumor evolution and the life expectancy of the patient. However, postponing this intervention greatly entails the risk that the tumor will end up infiltrating other non-visceral structures and that excessive consumption of opioids produces tolerance and uncontrollable adverse effects, drastically reducing the success of the techniques. Therefore, patient survival should not be a limiting factor for sympatholysis, since it is often not very clear and depends on more factors. Neurolytic blockade can achieve a reduction in opioids and their secondary effects and, in many cases, improve the quality of life of patients during the period that its effect lasts, without thereby significantly increasing the complication rate serious.
PROPOSAL FOR INTERVENTIONAL ALGORITHM
Pain management associated with pancreatic cancer should be, as in any other type of cancer pain, a multidisciplinary management. This requires a close collaboration between the services of Medical Oncology, Radiotherapeutic Oncology, Surgery, Digestive, Palliative Care and Pain, since the evolution of the disease causes these patients to undergo different tests and treatments that can influence the decision making. Ultrasound endoscopy may be necessary not only at the time of diagnosis, but also in other situations throughout the disease, such as, for example, to diagnose complications. Therefore, a teamwork is needed to take advantage of this technique and to perform the neurolysis at that time if indicated in order to avoid further suffering to patients with more unnecessary interventional techniques. Other considerations to take into account are that some chemotherapeutic treatments can cause alterations of hemostasis, to be taken into account for the selection of the percutaneous approach, and that the use of radiotherapy can improve cancer pain, another reason to perform a multidisciplinary and orderly management. Finally, a psychological assessment of patients is advisable to control factors that may decrease pain threshold, such as associated distress, anxiety or depression, since they can influence on the VAS assessment and on the final decision of the neurolytic block.

Fig. 7. Interventional algorithm for the management of visceral pain associated with pancreatic cancer. VAS: visual analog scale; MEDD: morphine equivalent daily dose.
Following the conclusions of the previously described publications, an interventional treatment algorithm for patients with visceral pain associated with pancreatic cancer can be established (Figure 7). The standard procedure would be the posterior approach of the celiac plexus (classical approach), since today it continues being the one with the greatest scientific evidence, preferably transcrural and guided by fluoroscopy, due to its simplicity and speed. Ultrasound endoscopy would be indicated for neurolysis taking advantage of its diagnostic or therapeutic indication, for situations of inability to prone position, and as an alternative in case the posterior approach fails. Percutaneous ultrasound neurolysis would be reserved for selected cases, depending on the experience of the operator, since it is the technique that has fewer publications to date. We hope that in the future, studies allowing ultrasound-guided techniques, whether percutaneous or endoscopic ultrasound, will be published as the "reference method", since they allow the visualization of surrounding structures with real-time injection control. An alternative to the blockage of the celiac plexus is the conventional radiofrequency of the splanchnic nerves, indicated when there is tumor infiltration of the celiac trunk, important anatomical alterations or if the celiac plexus neurolysis fails. It is also possible to consider a combined neurolysis - neurolytic blockade of the celiac plexus associated with neurolytic block or radiofrequency of the splanchnic nerves -, as reflected in the study of Marra et al. 60, and as we have been performing in our usual clinical practice at HM hospitals, with the aim of increasing the success rate and duration of analgesia without increasing the incidence of complications. However, no enough comparative studies supporting this hypothesis are available so far (Figure 8).
Regarding the ideal time to perform the blockade, our team advocates a neurolysis that is not late despite the fact that the duration of the effect is limited, since we find a series of advantages in patients, such as larger pain management, an improvement in the quality of life, and a lower tolerance to opioids. As indicated by one of the main variables to perform the neurolysis, it is mandatory that the pain is severe, which according to the WHO is estimated at a VAS > 7. Patients with pancreatic cancer and severe pain who undergo neurolysis procedures should be previously treated with potent opioids, being able to establish a morphine equivalent daily dose (MEDD) above 90 mg to confirm the blockage decision, or below if the patient present significant side effects derived from opioids.
REFERENCES
1. Sirri E, Castro FA, Kieschke J, Jansen L, Emrich K, Gondos A, Holleczek B, Katalinic A, Urbschat I, Vohmann C, et al. Recent trends in survival of patients with pancreatic cáncer in Germany and the United States. Pancreas 2016; 45: 908-914. [ Links ]
2. Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cáncer pain in adults. Cochrane Database Syst Rev. 2011; CD007519. [ Links ]
3. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cáncer. Nat Rev Cáncer. 2011; 11: 695-707. [ Links ]
4. Ayuso C, Sánchez M, Ayuso JR, De Caralt TM, De Juan C. Diagnosis and staging of carcinoma of the pancreas. Radiologia 2006; 48: 283-94. [ Links ]
5. World Health Organization. Cáncer Pain Relief, 2nd ed., WHO: Geneva, Switzerland, 2006. [ Links ]
6. Waldman SD. Atlas of interventional pain management. Saunders (W.B.) Co Ltd, 6/2009. [ Links ]
7. Ogawa S, Suzuki H, Yazaki S, et al. A clinical study of volumes and concentrations of ethyl alcohol for splanchnic nerve block. Pain Clin 1991; 4: 37-42. [ Links ]
8. Rykowski JJ, Hilgier M. Efficacy of neurolytic celiac plexus block in varying locations of pancreatic cáncer: Influence on pain relief. Anesthesiology 2000; 92: 347-54. [ Links ]
9. Iwata K, Yasuda I, Enya M, Mukai T, Nakashima M, Doi S, et al. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc 2011; Apr 23(2). [ Links ]
10. Yuen TST, Ng KFJ, Tsui SL. Neurolytic celiac plexus block for visceral abdominal malignancy: Is prior diagnostic block warranted? Anaesth Intensive Care 2002; 30: 442-8. [ Links ]
11. Herrero Trujillano M, Insausti Valdivia J, Mendiola de la Osa A, Pellejero Collado EM. An update of the management of antiplatelet and antithrombotic therapy for interventional technics in chronic pain. Rev Soc Esp Dolor, vol. 22 no. 3 Madrid may/jun 2015. [ Links ]
12. Kappis M. Erfahrungen mit Lokalansthesie bei Bauchoperationen. Verh Dsch Ges Cire 1914; 43: 87. [ Links ]
13. Meare DC, Bush WH, Bumett LL. Celiac plexus blochik: A roenrgenographic, anatomic study of technique and spread of solution in patients and corpses. Anesth Anal 1981; 60: 369. [ Links ]
14. Ischia S, Luzzani A, Ischia A, Faggion S. A new approach to the neurolytic block of the coeliac plexus: The transaortic technique. Pain 1983; 16: 333-41. [ Links ]
15. Ina H, Kitoh T, Kobayashi M, Imai S, Ofusa Y, Goto H. New Technique for the neurolytic coeliac plexus block: The transintervertebral disc approach. Anesthesiology 1996; 85: 212-17. [ Links ]
16. Plancarte-Sánchez R, Máyer-Rivera F, del Rocío Guillén Núñez M, Guajardo-Rosas J, Acosta-Quiroz CO. Transdiscal percutaneous approach of splanchnic nerves. Cir Cir. 2003; 71: 192-203. [ Links ]
17. Mercadante S, Klepstad P, Kurita GP, Sjøgren P, Giarratano A. On the behalf of The European Palliative Care Research Collaborative (EPCRC). Sympathetic blocks for visceral cáncer pain management: A systematic review and EAPC recommendations. Crit Rev Oncol Hematol 2015; Dec; 96(3): 577-83. [ Links ]
18. Mercadante S. Celiac plexus block versus analgesics in pancreatic cáncer pain. Pain 1993; 52: 187-192. [ Links ]
19. Lillemoe K, Cameron JL, Kaufman HS, Yeo C, Pitt H, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cáncer. A prospective randomized trial. Ann Surg. 1993; 217: 447-457. [ Links ]
20. Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K. Namiki A. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cáncer pain. Pain 1996; 64: 597-602. [ Links ]
21. Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double-blind trial of neurolytic celiac plexux block in patients with pancreatic cáncer. Br J Cáncer, 1998; 85: 199-201. [ Links ]
22. Staats PS, Hekmat H, Sauter P, Lillemoe K. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cáncer: a double-blind, randomized, placebo-controlled study. Pain Med 2001; Mar 1;2(1): 28-34. [ Links ]
23. Okuyama M, Shibata T, Morita T, Kitada M, Tukahara Y, Fukushima Y, Ikeda K, Fuzita J, Shimano T. A comparison of intraoperative celiac plexus block with pharmacological therapy as a treatment for pain of unresectable pancreatic cáncer. J Hepatobiliary Pancreat Surg. 2002; 9: 372-375. [ Links ]
24. Wong G, Schroeder D, Carns P, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cáncer: A randomized controlled trial. JAMA 2004; 291: 1092-9. [ Links ]
25. Jain PN, Shrikhande SV, Myatra SN, Sareen R. Neurolytic celiac plexus block: a better alternative to opioid treatment in upper abdominal malignancies: an Indian experience. J Pain Palliat Care Pharmacother, 2005; 19: 15-20. [ Links ]
26. Zhang CL, Zhang TJ, Guo YN, et al. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cáncer pain. Dig Dis Sci 2008; 53: 856-60. [ Links ]
27. Stefaniak T, Basinski A, Vingeerhoets A, Makarewicz W, Connor S, Kaska L, Stanek A, Kwienciska B, Lachinski AJ, Siedzinzki Z. A comparison of two invasive techniques in the management of intractable pain due to inoperable pancreatic cáncer: neurolytic celiac plexus block and videothoracoscopicsplanchnicectomy. Eur J Cáncer Surg. 2005; 31: 768-773. [ Links ]
28. Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, in- operable pancreatic cáncer. J Clin Oncol 2011; 29: 3541-3546. [ Links ]
29. De Oliveira R, Dor Reis MP, Prado WA. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cáncer pain. Pain 2004; Jul 110(1-2): 400-8. [ Links ]
30. Zhong W, Yu Z, Zeng JX, Lin Y, Yu T, Min XH, et al. Celiac plexus block for treatment of pain associated with pancreatic cáncer: A meta-analysis. Pain Pract. 2014; 14: 43-51. [ Links ]
31. Bhatnagar S, Khanna S, Roshni S, Goyal GN, Mishre S, Rana SP, Thulkar S. Early ultrasound-guided neurolysis for pain management in gastrointestinal and pelvic malignancies: an observational study in a tertiary care center of urban India. Pain Pract. 2012; Jan 12(1): 23-32. [ Links ]
32. Baig S, Moon JY, Shankaar H. Review of Sympathetic Blocks: Anatomy, Sonoanatomy, Evidence, and Techniques. Regional Anesthesia and Pain Medicine 2017; Vol 42, Number 3, May-June. [ Links ]
33. Ischia S, Ischia A, Polati E, et al. 3 posterior percutaneous celiac plexus block techniques -A prospective, randomized study in 61 patients with pancreatic-cáncer pain-. Anesthesiology 1992; 76: 534-40. [ Links ]
34. Tewari S, Agarwal A, Dhiraaj S, Gautam SK, Khuba S, Madabushi R, Shamshery C, Kumar S. Comparative Evaluation of Retrocrural versus Transaortic Neurolytic Celiac Plexus Block for Pain Relief in Patients with Upper Abdominal Malignancy: A Retrospective Observational Study. Indian J Palliat Care 2016; Jul-Sep 22(3): 301-306. [ Links ]
35. Marcy P, Magné N, Descamps B. Coeliac plexus block: utility of the anterior approach and the real time colour ultrasound guidance in cáncer patient. Eur J Surg Oncol. 2001; 27: 746-749. [ Links ]
36. Radpay B, Farhadi K, Radpay MZ, Goldstah A, Dabir S, Parsa T, Karam MB, Fathi M. Comparison between CT-scan and trans-abdominal sonography in celiac and splanchnic plexus blocks in patients with advanced pancreatic head cáncer. Tanaffos 2009; 8: 51-57. [ Links ]
37. Wyse JM, Chen Y, Sahai A. Celiac plexus neurolysis in the management of unresectable pancreatic cáncer: When and how? World J Gastroenterol 2014; March 7 20(9): 2186-2192. [ Links ]
38. Luz L, Al-Haddad M, DeWitt J. EUS-guided celiac plexus interventions in pancreatic cáncer pain: an update and controversies for the endosonographer, Endosc Ultrasound 2014; Oct 3(4): 213. [ Links ]
39. Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cáncer pain: a meta-analysis and systematic review. Dig Dis Sci 2009. [ Links ]
40. Fujii-Lau LL, William WR, Eldrige JS, Chari ST, Gleeson FC, Abu-Dayyeh BK, Clain JE, Pearson RK, Petersen BT, Rajan E, Topazian MD, Vege SS, Wang KK, Wiersema MJ, Levy MJ. Impact of celiac neurolysis on survival in patients with pancreatic cáncer. Gastrointest Endosc 2015; July 82(1): 46-56.e2. [ Links ]
41. Ascunce G, Ribeiro A, Reis I, Rocha-Lima C, Sleeman D, Merchan J, Levi J. EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pan- creatic malignancy (with video). Gastrointest Endosc 2011. [ Links ]
42. Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy 2012. [ Links ]
43. Fujii L, Clain JE, Morris JM, Levy MJ. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy 2012; 44 Suppl 2. [ Links ]
44. Mittal MK, Rabinstein AA, Wijdicks EF. Acute spinal cord infarction following endoscopic ultrasound-guided celiac plexus neurolysis. Neurology 2012; 78: e57-e59. [ Links ]
45. Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999; Apr 94(4): 900-5. [ Links ]
46. Santosh D, Lakhtakia S, Gupta R, Reddy DN, Rao GV, Tandan M, Ramchandani M, Guda NM. Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment Pharmacol Ther 2009; 29: 979-984. [ Links ]
47. Nobre Moura R, Guimaraes Hourneaux de Moura E, Marques Bernardo WM, Otoch JP, Castillo Bustamante FA, Vieira Albers DM, Rodelda Silva GL, Marques Chaves D, Luiz de Almeida Artifon E. Endoscopic-ultrasound versus percutaneous-guided celiac plexus block for chronic pancreatitis pain. A systematic review and meta-analysis. Rev Gastroenterol Peru. 2015; 35(4): 333-41. [ Links ]
48. Masuda T, Kuramoto M, Shimada S, Ikeshima S, Yamamoto K, Nakamura K, Baba H. Splanchnicectomy for pancreatic cáncer pain. Biomed Res Int. 2014; 2014: 941726. [ Links ]
49. Bhutiani N, Cheadle GA, Bahr MH, Vitale GC. Comparative efficacy of bilateral thoracoscopic splanchnicectomy for intractable pain secondary to pancreatic cáncer vs chronic pancreatitis. J Am Coll Surg 2017; Apr 224(4): 566e71. [ Links ]
50. Cosman ER. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med 2005; 6(6): 405-24. [ Links ]
51. Raj P, Sahinder B, Lowe M. Radiofrequency lesioning of splanchnic nerves. Pain Pract. 2002; 2: 241-247. [ Links ]
52. Garcea G, Thomasset S, Berry DP, Tordoff S. Percutaneous splanchnic nerve radiofrequency ablation for chronic abdominal pain. ANZ J Surg. 2005; 75: 640-644. [ Links ]
53. Papadopoulos D, Kostopanagiotou G, Batistaki C. Bilateral Thoracic Splanchnic Nerve Radiofrequency Thermocoagulation for the Management of End-Stage Pancreatic Abdominal Cáncer Pain. Pain Physician. 2013; Mar-Apr 16(2): 125-33. [ Links ]
54. Plancarte R, Guajardo-Rosas J, Reyes-Chiquete D, Chejne-Gómez F, Plancarte A, González-Buendía NI, et al. Management of chronic upper abdominal pain in cancer: transdiscal blockade of the splanchnic nerves. Reg Anesth Pain Med. 2010;35(6):500-6. DOI: 10.1097/AAP.0b013e3181fa6b42. [ Links ]
55. Ahmed A, Arora D. Fluoroscopy-guided Neurolytic Splanchnic Nerve Block for Intractable Pain from Upper Abdominal Malignancies in Patients with Distorted Celiac Axis Anatomy: An Effective Alternative to Celiac Plexus Neurolysis -A Retrospective Study. Indian J Palliat Care 2017; Jul-Sep 23(3): 274-281. [ Links ]
56. Shwita A, Amr Y, Okab M. Comparative Study of the Effects of the Retrocrural Celiac Plexus Block Versus Splanchnic Nerve Block, C-arm Guided, for Upper Gastrointestinal Tract Tumors on Pain Relief and the Quality of Life at a Six-month Follow Up. Korean J Pain 2015; January Vol. 28, No. 1: 22-31. [ Links ]
57. Amr SA, Reyad RM, Othman AH, Mohamad MF, Mostafa MM, Alieldin NH, Hamed FA. Comparison between radiofrequency ablation and chemical neurolysis of thoracic splanchnic nerves for the management of abdominal cáncer pain, randomized trial. Eur J Pain. 2018; Jul 5. [ Links ]
58. Puylaert M, Kapural L, Van Zundert J, Peek D, Lataster A, Mekhail N, Van Kleef M, Keulemans Y. Pain in Chronic Pancreatitis. Pain Practice 2011; Volume 11, Issue 5, 492-505. [ Links ]
59. Özyalçin NS, Talu GK, Camlica H, et al. Efficacy of coeliac plexus and splanchnic nerve blockades in body and tail located pancreatic cáncer pain. Eur J Pain 2004; 8: 539-45. [ Links ]
60. Marra V, Debernardi F, Frigerio A, Menna S, Musso L, Di Virgilio MR. Neurolytic block of the celiac plexus and splanchnic nerves with computed tomography. The experience in 150 cases and an optimization of the technic. Radiol Med. 1999; Sep 98(3): 183-8. [ Links ]
61. Johnson CD, Berry DP, Harrisetal S. An open randomized comparison of clinical effectiveness of protocol-driven opioid analgesia, celiac plexus block or thoracoscopic splanchnicectomy for pain management in patients with pancreatic and other abdominal malignancies. Pancreatology 2009; vol. 9, no. 6, pp. 755-763. [ Links ]
62. Molloy R, Benzon H. Neurolytic blocking agents: Uses and complications. In: Raj's Practical Management of Pain. Mosby Elsevier, Philadelphia, PA, 2008, 839-850. [ Links ]
63. Koyyalagunta D, Engle MP, Yu J, Feng L, Novy DM. The effectiveness of alcohol versus phenol based splanchnic nerve neurolysis for the treatment of intra-abdominal cáncer pain. Pain Physician 2016; 19(4): 281e92. [ Links ]
64. LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, Johnson C, Howard TJ, Lillemoe KD, DeWitt J. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cáncer: one injection or two? Gastrointest Endosc. 2011; Dec 74(6): 1300-7. [ Links ]
65. Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol 2009; 104: 326-329. [ Links ]
66. Lu F, Dong J, Tang Y, Liu H, Song L, Zhang K. Bilateral vs. unilateral endoscopic ultrasound-guided celiac plexus neurolysis for abdominal pain management in patients with pancreatic malignancy: a systematic review and meta-analysis. Support Care Cáncer 2018; Feb 26(2): 353-359. [ Links ]
67. De Cicco M, Matovic M, Balestreri L, Fracasso A, Morassut S, Testa V. Single-needle Celiac Plexus Block: Is Needle Tip Position Critical in Patients with No Regional Anatomic Distortions? Anesthesiology DEC 1997; 87(6): 1301-1308. [ Links ]
68. Bhatnagar S, Joshi S, Rana SP, Mishra S, Garg R, Ahmed SM. Bedside ultrasound-guided celiac plexus neurolysis in upper abdominal cáncer patients: a randomized, prospective study for comparison of percutaneous bilateral paramedian vs unilateral paramedian needle-insertion technique. Pain Pract. 2014; Feb 14(2): E63-8. [ Links ]
69. Gleeson FC, Levy MJ, Papachristou GI, Pelaez-Luna M, Rajan E, Clain JE, Topazian MD. Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy 2007; 39: 620-624. [ Links ]
70. Ha TI, Kim GH, Kang DH, Song GA, Kim S, Lee JW. Detection of celiac ganglia with radial scanning endoscopic ultrasonography. Korean J Intern Med 2008; 23: 5-8. [ Links ]
71. Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T, Ryozawa S, Hara K, Itoi T, Hanada K, Yamao K. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy 2013; 45: 362-369. [ Links ]
72. Bang JY, Sutton B, Hawes RH, Varadarajulu S. EUS-guided celiac ganglion radiofrequency ablation versus celiac plexus neurolysis for palliation of pain in pancreatic cáncer: a randomized controlled trial. Gastrointest Endosc. 2018; August 15. [ Links ]
73. Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. Celiac plexus neurolysis for abdominal cáncer pain: a systematic review. Pain Med 2013; Aug 1; 14(8): 1140e63. [ Links ]
74. McGreevy K, Hurley RW, Erdek MA, Aner MM, Li S, Cohen SP. The effectiveness of repeat celiac plexus neurolysis for pancreatic cáncer: a pilot study. Pain Pract 2013; Feb 13(2): 89-95. [ Links ]
75. Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic pain one year follow-up. Clin J Pain 2013; Sep 29(9): 807-13. [ Links ]
76. Amr YM, Makharita MY. Neurolytic sympathectomy in the management of cáncer pain-time effect: a prospective, randomized multicenter study. J Pain Symptom Manage 2014; Nov 48(5): 944-56.e2. [ Links ]
77. Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F. ESMO Guidelines Working Group. Management of cáncer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012. [ Links ]
78. Vissers KC, Besse K, Wagemans M, Zuurmond W, Giezeman MJ, Lataster A, et al. 23. Pain in patients with cáncer. Pain Pract. 2011; 11: 453-75. [ Links ]
79. Oh TK, Lee WJ, Woo SM, Kim NW, Yim J, Kim DH. Impact of celiac plexus neurolysis on survival in patients with unresectable pancreatic cáncer: a retrospective, propensity score matching analysis Pain Physician 2017; Mar 20(3): E357e65. [ Links ]
80. Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003; 57: 923-30. [ Links ]
Received: November 08, 2018; Accepted: June 29, 2019











 text in
text in